Question
Question: Why is the nitrogen's lone pair part of \( s{p^2} \) orbital in pyridine but part of p orbital in py...
Why is the nitrogen's lone pair part of sp2 orbital in pyridine but part of p orbital in pyrrole?
Solution
The hybridized sp2 orbital contains three orbitals and hybridized sp3 contains four orbitals. We can understand the reason by deep symmetry analysis and also considering the general aspects of the VSEPR theory.
Complete answer:
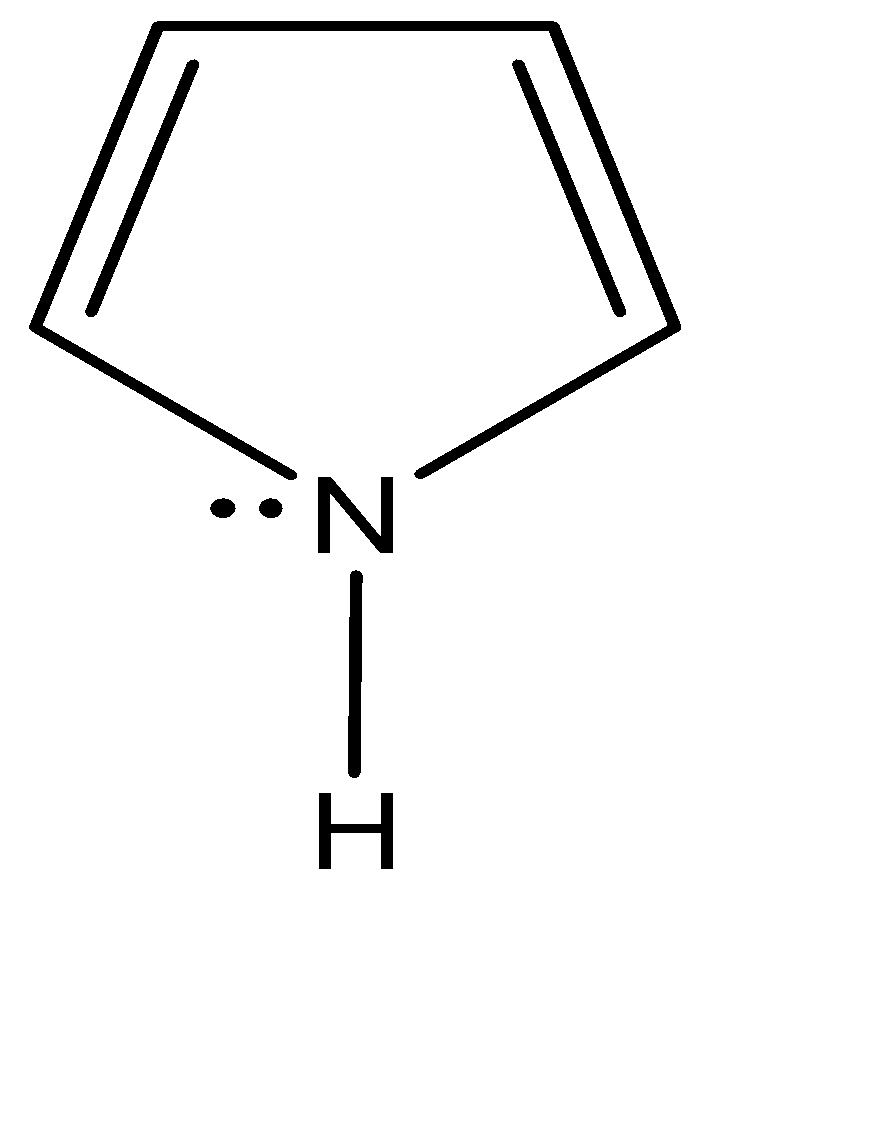
Pyrrole, is a five membered ring having the same no. of pi electrons as that of pyridine which is a six membered ring. Due to this there is an unusual extra fourth electron group on the Pyrrole’s Nitrogen. For the purpose of energetic stability i.e., aromaticity, Pyrrole has a sp2 hybridization, despite having four electron groups. The 2py orbital remains unhybridized, so it allows it to delocalize the electron density throughout the ring. For the hydrogen to be attached to the Pyrrole ring, the sp2 ring must align itself towards the hydrogen to overlap and form a bond. Therefore, the unhybridized 2py holds the lone pair of electrons.
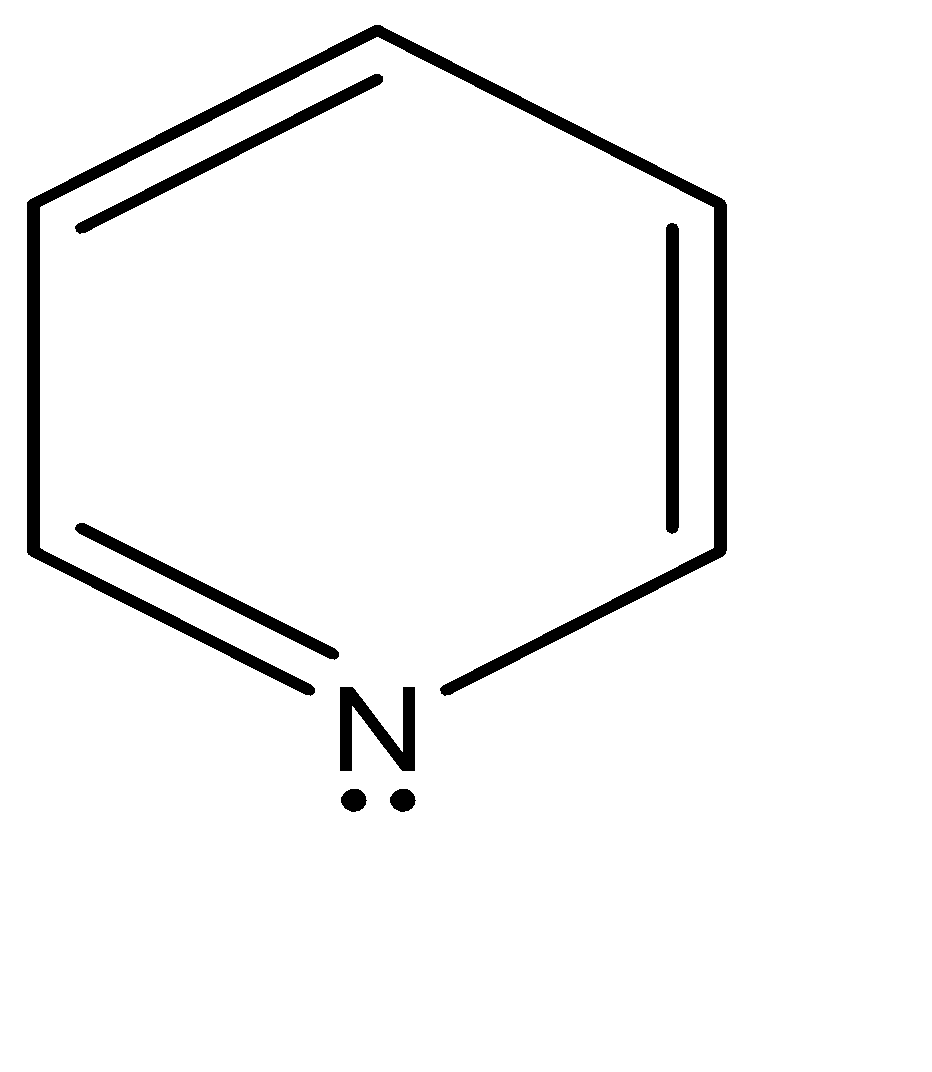
In pyridine the nitrogen has three electron groups only, therefore has the True sp2 hybridization. The lone pair is present on the third non bonding sp2 orbital. Since sp2 is the wrong symmetry, the electrons cannot delocalize the electron density throughout the ring. As a result, the lone pair sticks out of the ring as shown below:
In pyrrole, the nitrogen has four electron groups but the ring constraints and the hope for aromaticity make it energetically more favourable to have the ideal sp2 hybridisation instead of sp3 . The third sp2 orbital is used to bond with the hydrogen. If pyrrole had sp3 hybridisation, it could still bond with the hydrogen but the delocalisation of the electron density would not have taken place. So, it couldn’t be aromatic, hence pyrrole has a sp2 hybridisation only. The fourth orbital on pyrrole holds the lone pair which helps in delocalisation of electron density due to symmetry compatibility.
Note:
The fourth orbital will not hybridize unless the aromaticity is broken. Since aromaticity is the driving factor for the stability of pyrrole, it doesn’t hybridize and holds the lone pair of electrons and promotes delocalisation.
