Question
Question: Why is the melting point of benzamide more than acetamide?...
Why is the melting point of benzamide more than acetamide?
Solution
In the given question firstly we have to define the structure, shape and the nature of both the amides. This will help us to decide the factors that will make the difference in the melting point of both compounds. Now as we know that the benzamide is much stable and much heavier than the acetamide that makes it much more thermally stable in the direct comparison.
Complete step by step answer:
In the given question we have to first find out the structure and the formula of both the compounds so that we can compare in order to get the answer.
i.Benzamide :
-The formula of the given compound is C7H7NO
-The structure of the compound is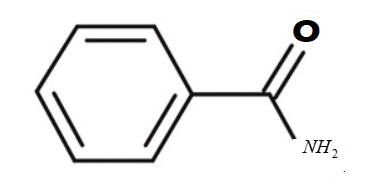
-It is an aromatic compound.
ii. Acetamide :
-The formula of the given compound is CH3CONH2
-The structure of the compound is
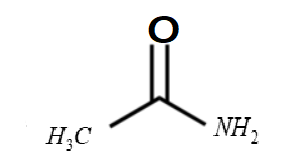
-It is a non aromatic compound.
Now by observing the nature, formula and the structure of the benzamide and acetamide we can state that, both of them contain the same functional group.But we can also observe that the molecular mass of benzamide is much greater than that of acetamide.
Therefore, it will result in benzamide having stronger intermolecular forces. This also emulates that it has higher melting point, due to the direct proportionality.
Note:
Benzamide is a white solid with the properties of an amide. It is the simplest amide derivative of benzoic acid. It is slightly soluble in water, and soluble in many organic solvents. A number of substituted benzamides exist.
