Question
Question: Why is the boiling point of ethyl bromide higher than that of ethyl chloride? 
Write the structure of X in the above reaction?
Solution
Haloalkanes are compounds consisting of an alkyl group attached to the halogen atom. The boiling point increases by increasing the Vander Waal forces. Ethyl bromide has stronger Vander Waal forces than ethyl chloride. The boiling point is also more in ionic compounds.
Complete answer:
Alkanes are saturated hydrocarbons consisting of only carbon and hydrogen atoms. Alkanes participate in substitution reactions only. haloalkanes are compounds consisting of halogens attached to an alkyl group.
The boiling point is more in the compound with strong Vander Waal forces.
In ethyl bromide, the bromine atom has higher electronegativity than chlorine. In ethyl bromide, the Vander Waal force is more than ethyl chloride. Thus, ethyl bromide has a higher boiling point than ethyl chloride.
Benzene is an aromatic compound with molecular formula C6H6 , it participates in substitution reactions but not in addition reactions.
When benzene reacts with acetyl chloride in presence of anhydrous aluminum trichloride the acetyl group substitutes one of the hydrogen atoms in benzene and forms acetyl benzene.
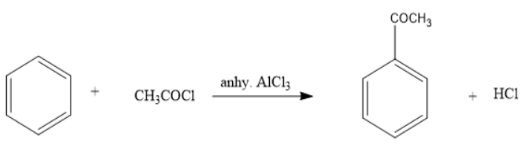
Thus, the compound X formed in the given reaction is acetyl benzene.
Note:
The atomic radius increases along with the groups. Chlorine and bromine belong to the same group in which chlorine has a smaller size than bromine. The compound consisting of atoms with more size has a maximum of Vander Waal forces. High Vander Waal forces result in a high boiling point.
