Question
Question: Why is that \(S{F_4}\) molecule, the lone pair of \({e^ - }\) occupy equatorial positions in prefere...
Why is that SF4 molecule, the lone pair of e− occupy equatorial positions in preference to axial positions? What is the shape of the molecule?
Solution
The sulphur tetrafluoride molecule consists of 5 regions with electron density around the central sulphur atom with four bond pairs and one lone pair. The bond angles formed between the atoms are 102∘ present between the equatorial fluorine atom. The bond angle present between the axial fluorine atoms is 173∘.
Complete answer:
The sulphur atom in sulphur tetrafluoride has an oxidation state of +4. Sulphur has six valence electrons in its outermost shell from which two electrons form a lone pair. By using the principle of VSEPR theory we can determine the shape of the molecule. The shape of the molecule is see-saw with the atom sulphur at the centre. This shape is known as disphenoidal. The geometry of the electron pair in this molecule is trigonal bipyramidal. The lone pairs of the molecule are positioned at equatorial position because they need more space than bond pairs due to repulsion. When the lone pair at equatorial position they form the bond angle of 120∘ while at axial position they form the angle of 90∘. So greater is the bond angle, lesser is the repulsion between the lone pair and bond pair. The structure of the molecule is the following:
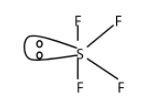
Note: Sulphur tetrafluoride is colorless gas which releases hydrogen fluoride when it is exposed to water or moisture. It is a useful reagent for the formation of organofluorine compounds that is widely used in pharmaceutical industries. This structure has four faces of a tetrahedron with the same perimeter.
