Question
Question: Why is phenoxide ion a stronger acid than alkoxide ion?...
Why is phenoxide ion a stronger acid than alkoxide ion?
Solution
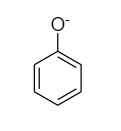
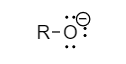
The conjugate base of phenol is a phenoxide ion. Phenoxide is a conjugate base, meaning it is made up of an acid that has lost its hydrogen. The hydrogen of the hydroxyl leaves, and an O− remains, forming the ‘oxide ion’ part of the phenoxide ion. When a hydrogen atom is removed from a hydroxyl group of an alcohol when it is interacted with a metal, an alkoxide is created. The formula for alkoxide is RO , where R is the organic substituent from the alcohol.
Complete answer:
The phenoxide ion is a weaker base than alkoxide ion as the phenoxide ion is resonance stabilized, and requires less solvation.
Because the charge is partially delocalized around the ring, the charge density of the phenoxide anion is substantially lower than that of an aliphatic alkoxide. The charge on alkoxide, on the other hand, is limited to the oxygen centre. When phenoxide is deprotonated, it causes less solvent ORDER, and its acidity is entropically favoured. As a result, rather than being an enthalpy phenomenon, this is an entropy phenomenon.
The ability of phenols to lose hydrogen ions and produce phenoxide ions gives them their acidity.
The benzene ring is sp2 hybridised carbon atom connected directly to the hydroxyl group that works as an electron-withdrawing group in a phenol molecule.
In comparison to the hydroxyl group, this sp2 hybridised carbon atom of a benzene ring linked straight to it has a stronger electronegativity.
The electron density on the oxygen atom lowers due to the higher electronegativity of this carbon atom compared to the hydroxyl group connected.
The structure of phenoxide ion and alkoxide ion are as follows:
Note:
Because charge separation occurs during resonance in the case of phenols, the phenoxide ion is more stable than phenols. An alkoxide is an acid's conjugate base Phenoxide ions contain resonance structures that are non-equivalent in which negative charge is there which is less effectively delocalised over less electronegative carbon atom and 1 oxygen atom.
