Question
Question: Why is phenol more acidic than ethyl alcohol?...
Why is phenol more acidic than ethyl alcohol?
Solution
We must need to know that a hydrogen ion is lost by Phenol to give rise to phenoxide ion that resonates and stabilized itself and this electrons are lost turns the phenol more acidic when compared to ethyl alcohol. We must remember that phenols as well as alcohols are weak acids. The acidity of alcohol is less when compared to phenols as it is highly hard to eliminate the hydrogen ion from alcohol.
Complete step by step answer:
For any compound, acidity is dependent on the capacity to liberate the ions of hydrogen. Thus, in the case of ethanol it is very hard to liberate ions of hydrogen to it. So, we could say that the acidity of ethanol is less when compared to phenol whereas on the other side, the acidity of phenol is more when compared to ethanol so it could the ion of hydrogen very easily due to the delocalization of electrons occurring in phenols by resonance. When a hydrogen ion is lost by phenol, it forms phenoxide that is then strengthened to certain extent as negative charge present on atom of oxygen that is delocalized around the ring means this negative charge is similarly spread through the compounds (or) we could say that is shared the number of carbon atoms present in the ring of benzene, so the more stable, the ion that resulted is the more likely is to form.
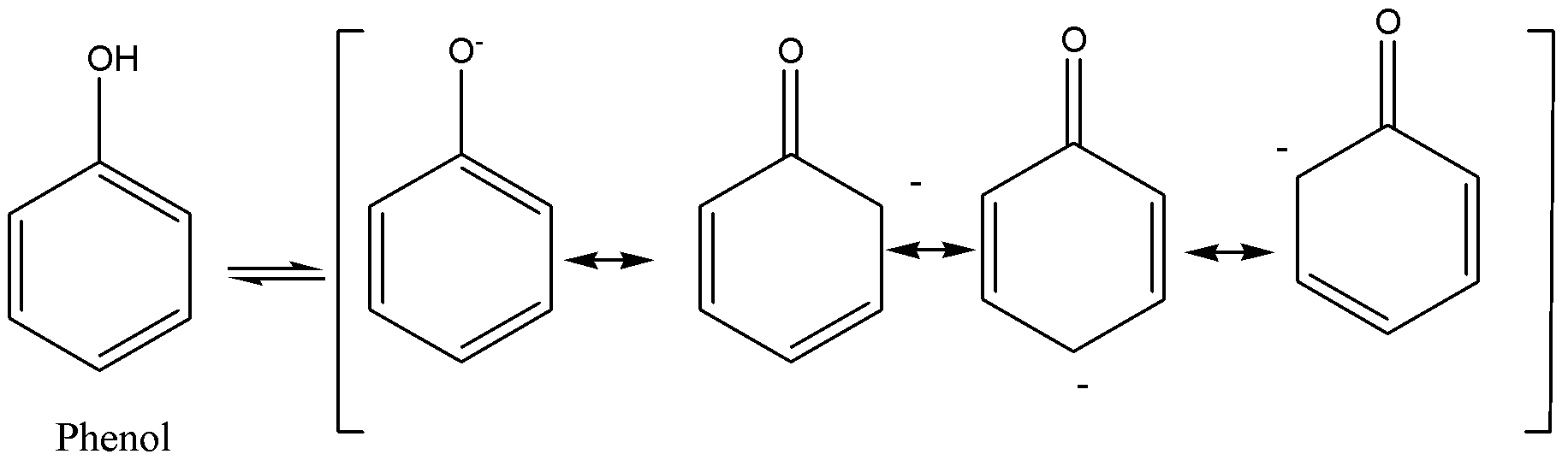
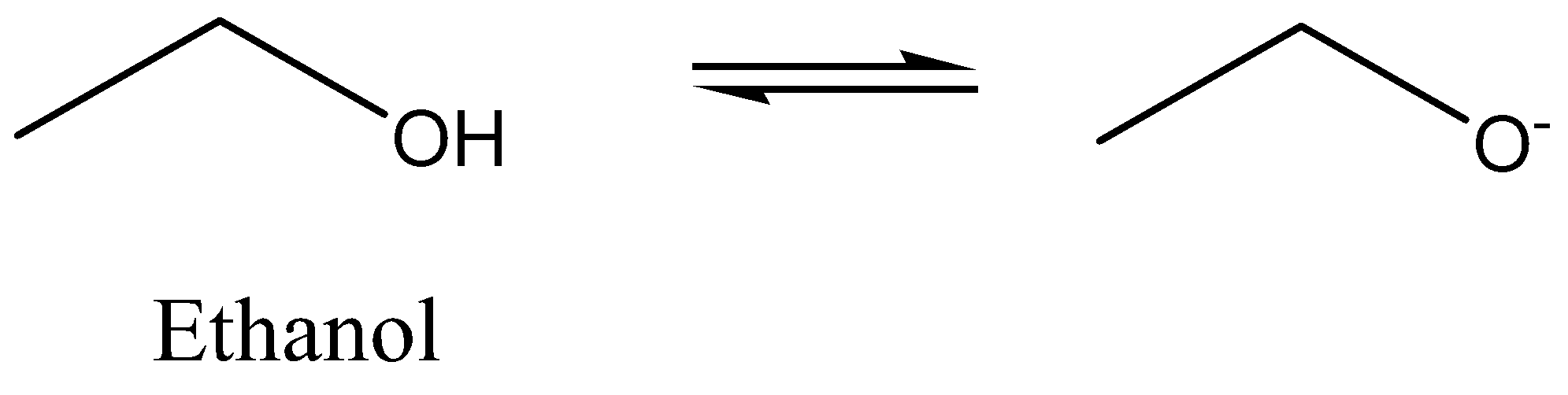
Another factor is that the presence of electron withdrawing groups raises the phenol’s acidity by strengthening the resulting ion of phenoxide and on the other end; the presence of electron releasing groups reduces the phenol’s acidity by de-strengthening the ion of phenoxide.
Note: We have to remember that the acidity is directly proportional to the strength of the conjugate base. In simple words, we can remember that resonance stabilization is undergone by phenoxide ion in phenol. So, its conjugate base is more stable when compared to ethanol. Electrons are drawn away from the reaction by the electron withdrawing group whereas the electrons are released in the centre of the reaction by the electron releasing group. Electron releasing groups are otherwise called electron donating groups.
