Question
Question: Why is \[Pb{{O}_{2}}\] not peroxide?...
Why is PbO2 not peroxide?
Solution
Peroxide ion has the molecular formula O22−. The treatment of metallic oxides with acid i.e. hydrochloric acid produces hydrogen peroxide. The formation of peroxides is feasible with hydrogen, alkali and alkaline earth metals.
Complete answer:
Peroxides are formed by the treatment of metallic oxides with acid i.e. hydrochloric acid. The molecular formula of peroxide ion is O22−. The structure of hydrogen peroxide is given in the following diagram:
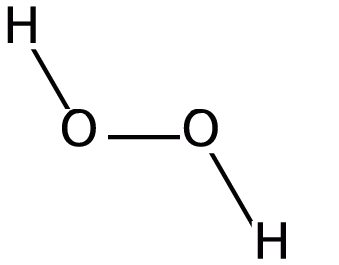
From the above diagram, we conclude that in peroxides, there is a single bond present between two oxygen atoms and charge on each oxygen atom is −1. Let us now consider the structure of PbO2 which is described in the following diagram-
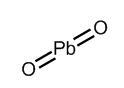
From the above structure, we conclude that O−Obond is not present in PbO2 . In PbO2 , lead is present in +4 oxidation state while each oxygen atom carries a −2 charge.
Therefore, PbO2 is not a peroxide.
Additional information:
The chemical name of PbO2 is lead dioxide. The electronic configuration of lead is [Xe]6s24f145d106p2. Considering the electronic configuration into the account, the oxidation state of lead should be +2 and +4. Due to the inert pair effect, +2oxidation state of lead is more stable in comparison to +4 . As a consequence, PbO is much more stable than PbO2.
Note:
It is important to note that the structure of PbO2 doesn’t contain O−O bond. Hence, PbO2 is not a peroxide. In PbO2 , lead is present in +4 oxidation state while each oxygen atom carries a −2 charge.
