Question
Question: Why is \({[Ni{(CN)_4}]^{2 - }}\) diamagnetic but \({[NiC{l_4}]^{2 - }}\) paramagnetic?...
Why is [Ni(CN)4]2− diamagnetic but [NiCl4]2− paramagnetic?
Solution
To solve this question, we need to understand the strength of the ligands. The strength of the ligands can be known from the spectrochemical series. According to that series Cl is a weak field ligand and CN is a strong field Ligand.
Complete answer:
The central metal atom in both the given complexes is Ni and Ni is in +2 Oxidation state in both the complexes. Nickel has a electronic configuration of: [Ar]3d84s2
In +2 oxidation state Nickel has the configuration as: Ni+2=3d8
Both the complexes have 4 ligands attached to it hence we can say that both the complexes have a coordination number = 4 . Coordination number 4 can have two possible geometries; tetrahedral and square planar. The difference is that d8 tetrahedral complexes tend to be high spin and square planar d8 tend to be low spin.
According to the spectrochemical series, Cl is a weak field ligand. It is a π donor. It promotes high spin complexes, because of the minimized repulsions and Crystal Field energy splitting. While filling of electrons it follows the Hund’s rule (i.e. one electron in each orbital and the doubling up). While CN− is a strong field ligand, it is a σ donor and π acceptor. It promotes low spin complexes, because it has maximum electron repulsion and crystal field splitting energy. It doesn’t follow the Hund’s rule for filling of electron (i.e., first pairing the electrons and then filling up the higher energy orbitals)
Consider the first complex given to us [Ni(CN)4]2−. The splitting of the d-orbitals according to crystal field theory can be given as:
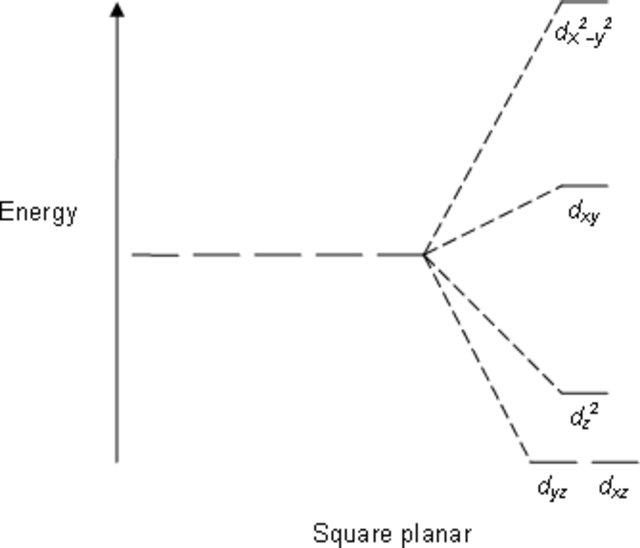
We have 8 electrons to fill in the orbitals. CN is a strong field ligand and gives a low spin configuration. As we fill the orbitals, the electrons get paired up and making two electrons each in dyz,dxz,dz2,dxy orbitals. As all the electrons are paired up, no unpaired electrons are present making the compound diamagnetic.
Next we have [NiCl4]2−. Cl is a weak field ligand and gives a high spin complex. The tetrahedral splitting of d-orbital can be given as:
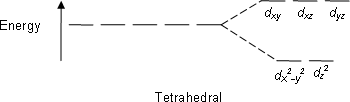
To fill in 8 electrons, we will start filling from the e orbitals. One electron in all the orbitals will fill in, and later the pairing up will take place. The electron count can hence be given as 2 each in dx2−y2,dz2,dxy and one electron each in dxz,dyz. There are thus two unpaired electrons, making the complex paramagnetic.
Note:
Remember that for d8 electronic configurations with C.N=4, if strong field ligands are present, always square planar complexes will be formed (because of pairing of electrons) and if weak field ligands are present then tetrahedral complexes will be formed.
