Question
Question: Why is iodine a better leaving group than other halogen atoms? A. Due to its small size. B. Du...
Why is iodine a better leaving group than other halogen atoms?
A. Due to its small size.
B. Due to its large size.
C. Due to its least electronegativity.
D.Due to its more electro positivity.
Solution
There are two kinds of substitution reactions. One is a nucleophilic substitution reaction and the other is an electrophilic substitution reaction. In the case of substitution of alkyl halide, the halide ion is the leaving group for the substitution reaction.
Complete step by step answer:
The leaving ability of the halide depends upon the C−X bond strength. The higher the bond stability of the C−X bond, the lower will be the leaving ability of the halide. In the case of halogen from fluorine to iodine size increases, therefore, the bond stability with the carbon decreases as with the increasing size of the halide the overlap of orbital decreases.
Therefore, in the case of iodine due to large size, the C−I bond stability decreases, and iodine becomes a very good leaving group than other halogen atoms.
So the correct option is B.
Additional information:
In the case of nucleophilic substitution reactions, there are mainly two kinds of substitution reactions: one is SN1 another one is SN2. Where 1 and 2 stand for the order of the reaction. Where the SN1 reaction rate depends upon the carbocation stability, which is formed from the alkyl halide, and SN2 the reaction rate depends upon the steric hindrance of the backside of the alkyl halide.
There is a graph representation of the order of rate of the reaction with the substrate of nucleophilic substitution reaction.
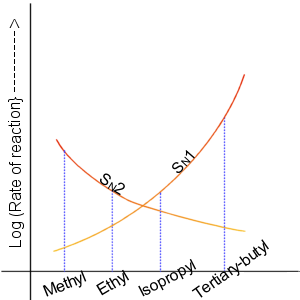
Note:
The SN2 reaction is a nucleophilic substitution reaction where one bond is broken, and another is formed simultaneously. The two reacting species are involved in the rate-determining step of the reaction. In the case of SN1 in the rate-determining step, carbocation is formed. only one reacting species is involved in the rate-determining step that is why it is a 1st order reaction. Substitution nucleophilic by the molecular reaction is also referred to as associative substitution and interchange mechanism.
