Question
Question: Why is carboxylic acid weaker than HCl?...
Why is carboxylic acid weaker than HCl?
Solution
The acidity or basicity of a chemical is going to depend on the capability of donation of H+ and OH− respectively. If the chemical is going to donate the H+ ions very easily then the chemical is called strong acid and vice versa.
Complete answer:
- In the question it is asked why carboxylic acid is weaker than HCl.
- First, we should know about the dissociation of the acid in water.
- The dissociation of the carboxylic acid in water is as follows.
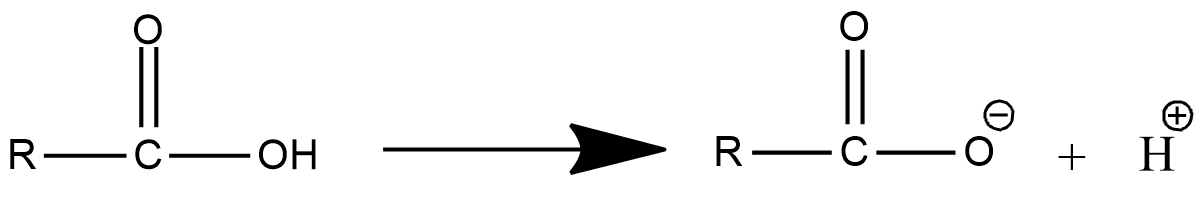
- In the above chemical dissociation of carboxylic acid, we can see that the carboxylic acid is going to convert into carboxylate anion and H+ ion.
- But the carboxylate anion is going to exist in the following resonance structures.
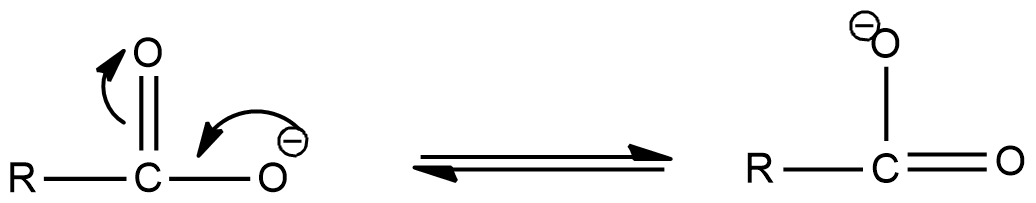
- Due to the existence of the above chemical structures the H+ ion formed at the time of dissociation is going to be grabbed by the formed resonance structures of the carboxylate anion.
- Coming to the dissociation of the HCl and it is as follows.
HCl→H++Cl−
- The formed H+ in the above chemical reaction is not going to be grabbed by the chloride ion which is formed in the above dissociation chemical reaction.
- Therefore, the carboxylate functional group is going to grab the H+ ion which is formed after its dissociation but the HCl is not going to do like this.
- Therefore, the carboxylic acid is a weak acid when compared to HCl.
Note:
The strength of an acid is going to depend on the capability of the donation of the H+ and not going to depend on the capability to accept the H+ which is formed by itself during the dissociation in water.
