Question
Question: Why is \(BrF_4^ - \) square planar, whereas \(BF_4^ - \) is tetrahedral?...
Why is BrF4− square planar, whereas BF4− is tetrahedral?
Solution
The geometry of square planar molecules describes the stereochemistry adopted by chemical compounds. The d-orbital splitting diagram for square planar (D4h)transition metal complexes is derived from the general octahedral (Oh)splitting diagram.
Complete answer:
In this question, bromine has 7 valence electrons in its ground state electronic configuration whereas boron has only 3 valence electrons in its ground state electronic configuration as given below:
For Br,
[Ar]4s23d104p5
For B,
[He]2s22p1
In these electronic configurations, we can see that the valence shell of bromine contains 7 electrons, 2 from the s subshell and 5 in the p subshell.
In the first case when Br is the central atom bonded with four F atoms, the four of the seven electrons of bromine form a bond with F atom. Here this shows that three electrons do not take part in bond formation. As there is a negative charge in the compound this indicates the additional electrons which will be paired with one of the three lone electrons.
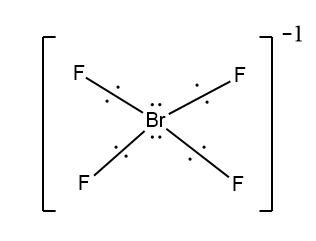
Here you can see that four F will align in a square plane and the lone pairs are on either side of the plane.
In the case of BF4−, boron only has three valence electrons, so three F will bond with boron and the fourth Fwill occupy the open p orbital of B. Here the extra electron shown in red is not present in the ground state configurations that provide the negative charge.
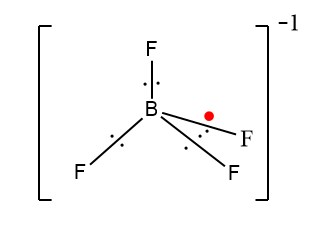
Thus, by these diagrams it is clear that BrF4− has square planar and BF4− has a tetrahedral shape.
Note:
In square planar, the constituent atoms surround the central atom which form the corners of a square on the same plane. Whereas in the tetrahedral, on the centre of the four substituents, there the central atom forming the corners of the tetrahedron.
