Question
Question: Why is bithionol added to soap?...
Why is bithionol added to soap?
Solution
HINT: Bithionol is an aromatic compound. It contains two aromatic rings bonded with a sulphur atom in between along with other groups like hydroxyl and chlorine. It is used in medicated soaps and it also reduces the consequence of bacterial decomposition on skin.
COMPLETE STEP BY STEP SOLUTION: We know that soap is a mixture of sodium or potassium salts of various naturally occurring fatty acids. It is used in a variety of cleansing and lubricating products. Soap is created by mixing of fats and oils with a base whereas a detergent is made by combining chemical compounds in a mixer.
When we use soap for cleaning, it solubilizes the dirt particle which then can be separated and hence leaves the substance clean.
When we use soap as a surfactant in hand washing, it lathers with a little water and kills microorganisms by destroying their membrane lipid bilayer and denaturing their proteins.
It also emulsifies oils and hence enables them to be carried away under running water.
Now let us discuss why bithionol is added in soaps.
Bithionol acts as an antibacterial, anthelmintic and algaecide. It has two aromatic rings with a sulphur atom bonded between them and to the phenyl ring multiple chlorine and hydroxyl groups are attached. These functional groups are capable of polar as well as hydrophobic and ionic interactions.
We can draw its structure as-
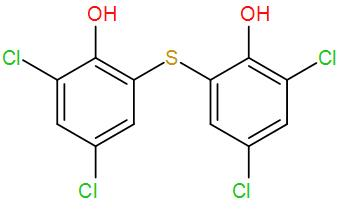
Bithionol is an aromatic compound and it is added to soaps as a disinfectant. It also reduces the odour which is produced by the bacterial decomposition of organic matter present on our skin. This is the required answer.
NOTE: Bithionol was formerly added in soaps as antiseptic and also in cosmetics but the US FDA banned it due to its photosensitizing effects. Photosensitivity is the amount to which an object reacts upon receiving photons like from visible light. Bithionol was found to cause photocontact sensitization.
