Question
Question: Why is beta decay continuous?...
Why is beta decay continuous?
Solution
We need to know that β decay is not a continuous process but the kinetic energy spectrum of the β decay emitted electrons is continuous. A β decay process is a type of radioactive decay where an electron is emitted from an atomic nucleus along with an electron antineutrino.
Complete answer:
To solve this problem we can write the β decay of carbon 14 as,
614C→714N+e−+ve
If we plot a fraction of electrons having a given kinetic energy against that energy.
Here the X axis represents the electronic kinetic energy and Y-axis represents the energy of that kinetic energy.
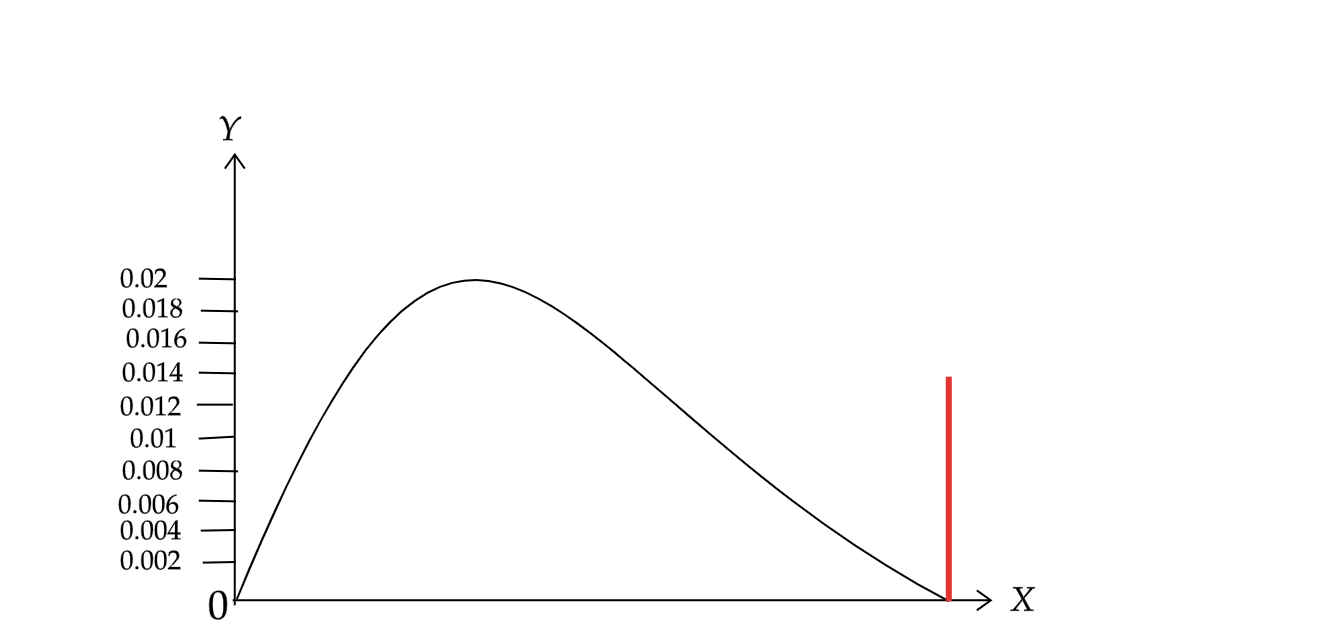
Since from the above equation we can say that the electors are emitted as a stream of discrete particles, β decay is not continuous.
Hence we can say that these emitted beta particles have a continuous kinetic energy spectrum. And the energy range is from zero to maximum with available energy is Q. If we carried away only the electrons then the graph would look like the red line as given in the above figure at the right of the graph. Instead we get the continuous energy spectrum in blue. This continuous energy spectrum occurs only when the Q is shared between the electrons and the antineutrino.
Note:
Keep in mind that typical Q is around the kinetic energy of about 1MeV, but it can range from a few KeV to a few tens of MeV. Remember that the rest mass energy of the electrons is about 511keV, and most of the energetic β particles have a speed close to the speed of light.
