Question
Question: Why is benzene an aromatic hydrocarbon?...
Why is benzene an aromatic hydrocarbon?
Solution
Benzene is defined as an aromatic compound which is composed of only carbon and hydrogen atoms therefore it is also known as aromatic hydrocarbon. Aromaticity of any compound is determined by applying Huckle’s rule which states that if any compound possesses (4n+2)π electrons which have capability to delocalize are known as aromatic compounds.
Complete answer:
As we know, the molecular formula of benzene is C6H6 . According to the basic concept of orbital theory, all the 6 carbon atoms of benzene are sp2 hybridized. When a carbon atom undergoes overlapping, out of three hybrid orbital of the carbon atom two undergoes axillary overlapping with the adjacent carbon atoms to form the bond. While the remaining last hybrid orbital of carbon undergoes overlapping with the hydrogen atom to form a bond with it. All the carbon atoms of benzene attached to one hydrogen atom to complete its valency to 4 . So, the final structure of benzene will be:
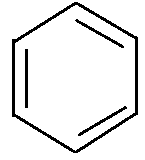
In order to calculate the aromaticity of a compound we begin with determining the number of electrons present in it. After this, put the value of (π) electrons in Huckle’s formula. If the final answer is a whole number then the compound must be aromatic.
From the above structure we find that there are a total 6πelectrons present in benzene. On applying Huckle’s rule we get,
(4n+2)=6
4n=6−2
After solving this we get
n=44
n=1
Since, the value of (n) is a whole number, benzene is an aromatic compound.
Note:
According to Huckle’s rule a compound is said to be aromatic only if it satisfies some basic requirement like the compound must be planar in nature, cyclic, and have (4n+2)π delocalized electrons, presence of conjugation within molecule. Compounds which are non-planar are considered as non-aromatic compounds like annulenes.
