Question
Question: Why does p-nitro chlorobenzene undergo displacement reactions readily with an attack of nucleophilic...
Why does p-nitro chlorobenzene undergo displacement reactions readily with an attack of nucleophilic HO ions?
Solution
There are two kinds of substitution reactions. One is a nucleophilic substitution reaction and the other is an electrophilic substitution reaction. In the case of nucleophilic substitution reactions, there are mainly two kinds of substitution reactions: one is SN1 another one is SN2. Where 1 and 2 stand for the order of the reaction.
Complete step by step answer:
In the case of p-nitro chlorobenzene due to the presence of the nitro group, the electron density of the benzene group gets reduced due to the −R effect of the nitro group. Due to the lower electron density, the nucleophilic attack of HO the group is favored. As a result, the substitution of the chlorine HO takes place. The reaction is shown below,
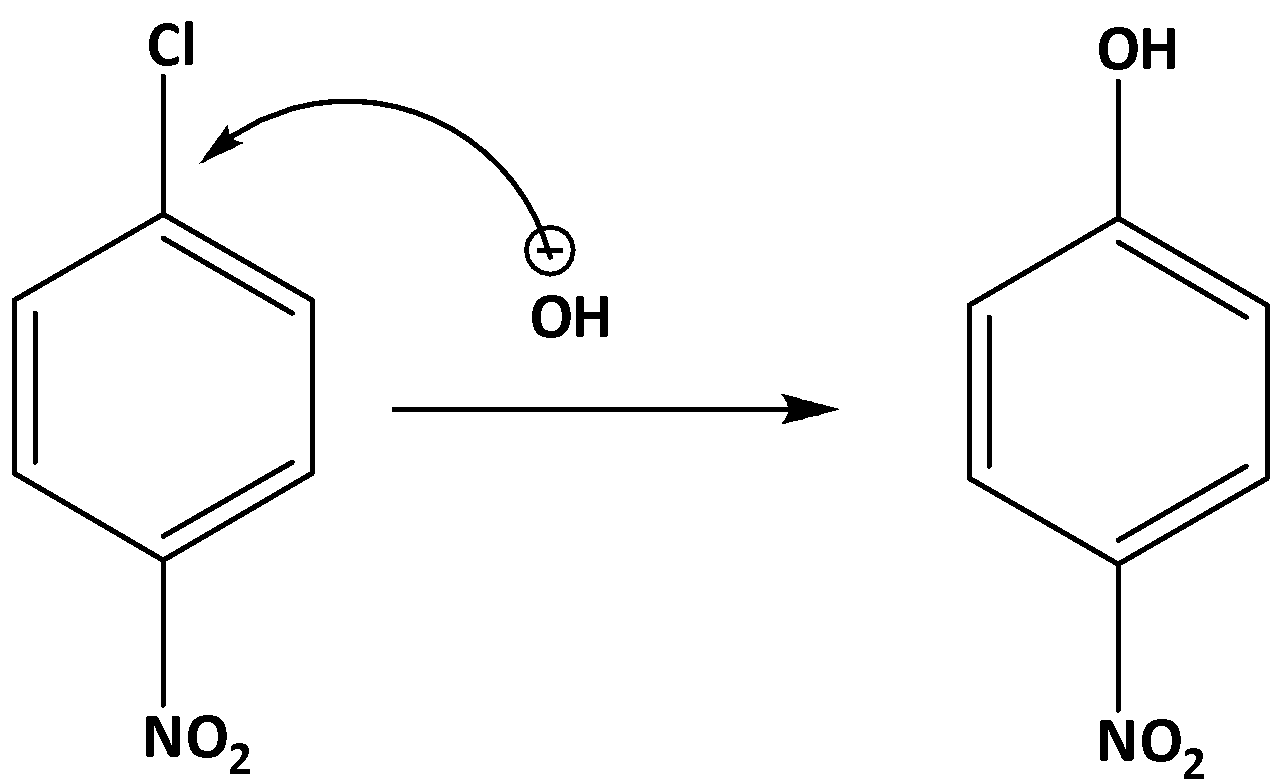
Additional information:
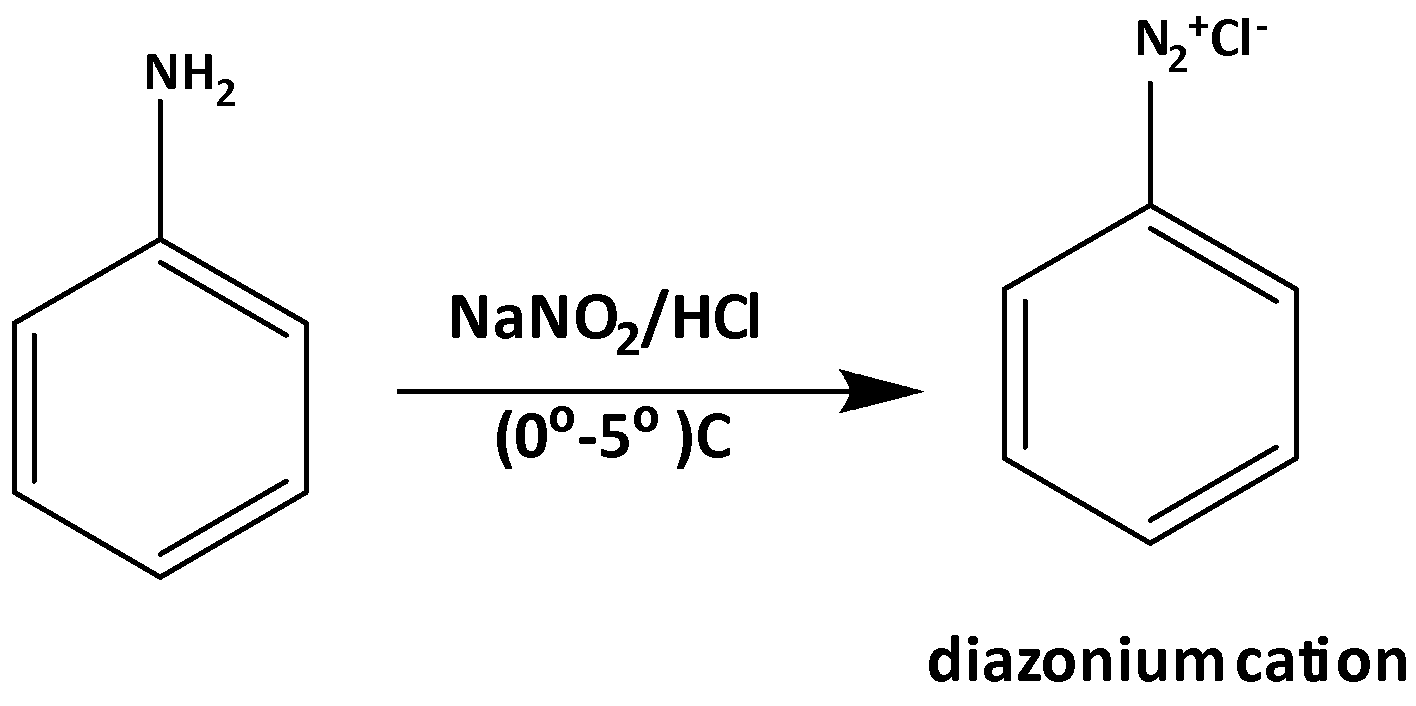
There are so many different kinds of reaction sequence pathways to convert 3-Methylaniline into 3-nitrotoluene. One of those is using diazonium cation formation reaction as one reaction of the sequence. This diazonium formation is a very common reaction from class XII organic chemistry.
Now the diazonium formation reaction is shown below,
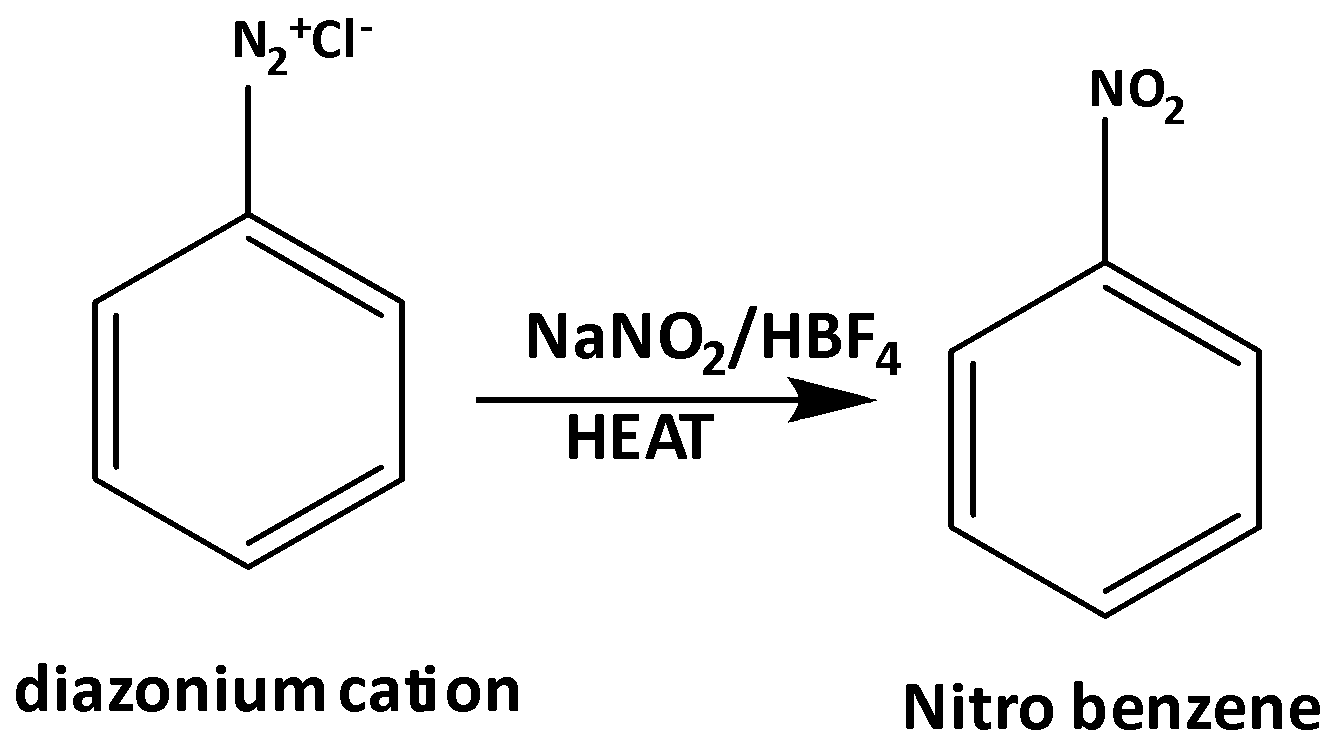
From the diazonium cation, nitrobenzene can be produced very easily. To convert diazonium cation nitrobenzene the following process can be used.
Now using these two reactions the conversion of 3-Methylaniline to 3-nitrotoluene is possible as follows.
Note:
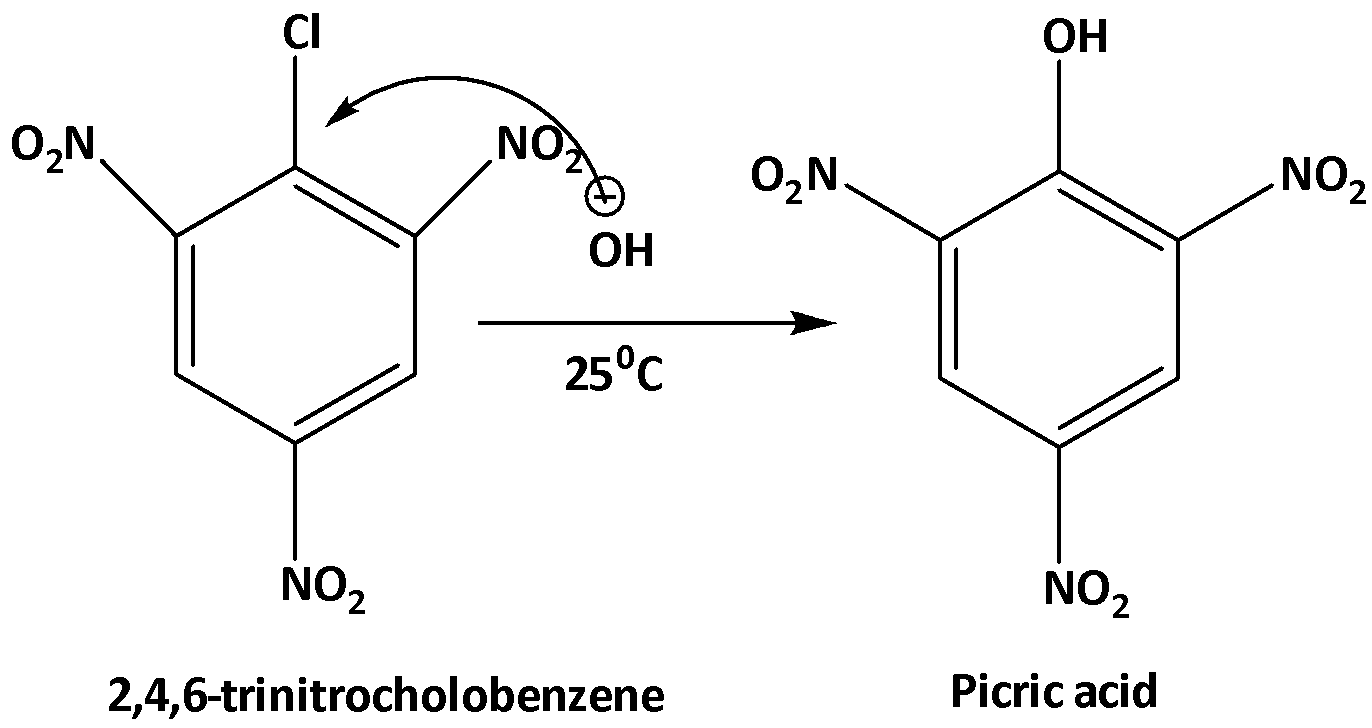
With increasing the number of the nitro group in the benzene ring this substitution reaction becomes faster. In the case of 2,4-dinitrochlorobenzene the substitution reaction by HO gets faster. This is because the electrophilicity of the benzene ring becomes greater than before which facilitates this reaction. And in the case of 2,4,6-trinitrochlorobenzene, this substitution reaction occurs at room temperature and forms picric acid. The reaction is shown below.