Question
Question: Why does \({ NO }_{ 2 }\) dimerise?...
Why does NO2 dimerise?
Solution
Look at the valence electrons of the central atom of NO2 molecule along with the bonding that takes place in the molecule. By the type of bond we can calculate the number of electrons that are involved in the formation of that bond.
Complete step by step solution:
On dimerization of NO2, N2O4 is produced. This dimerization takes place due to the formation of new covalent bonds between two molecules of NO2.
Let us look at the structure of NO2.
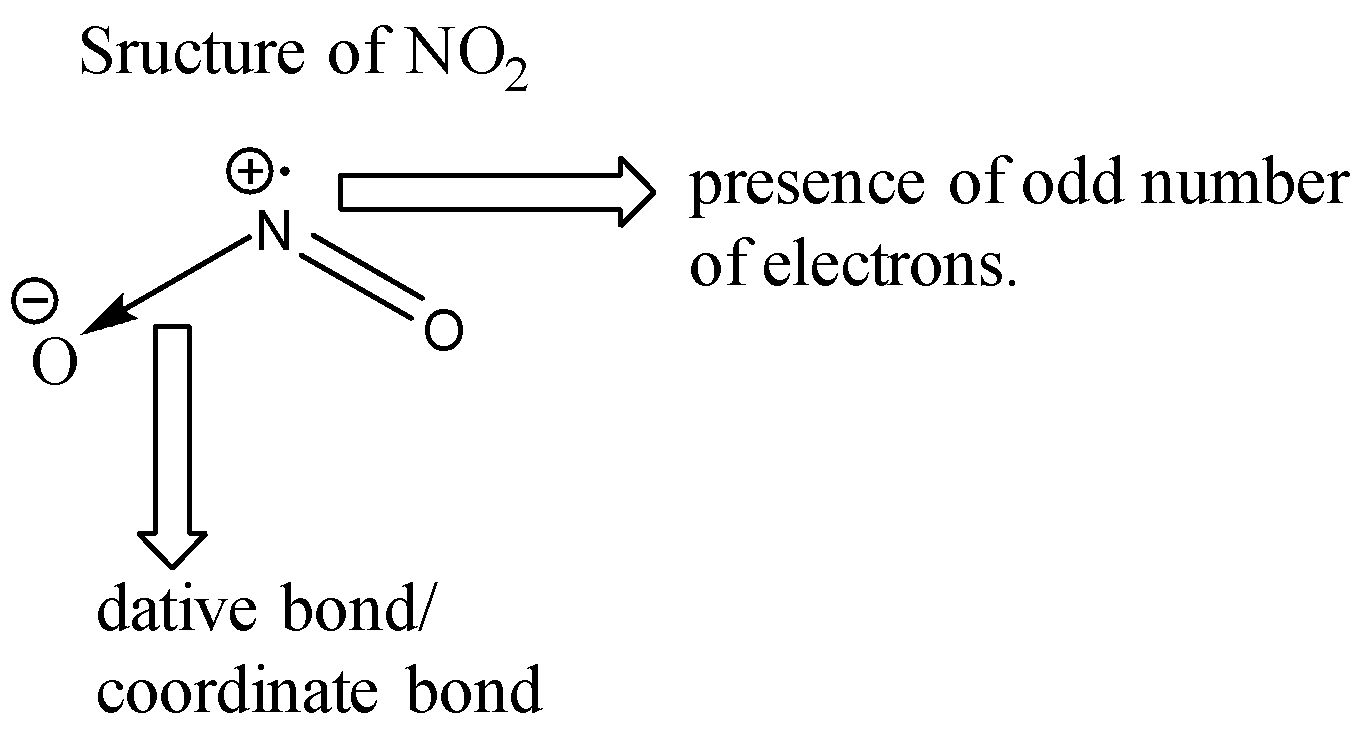
Nitrogen has a total of 5 valence electrons. 2 of these electrons are given away to an oxygen atom for the formation of a coordinate bond. Two electrons are used for forming double bonds with another oxygen atom. One electron is left behind unpaired (free radical) due to which the molecule of NO2 is highly unstable. Since each molecule of NO2 has an unpaired electron, when these two molecules combine together, a bond formation takes place and we get a new molecule of N2O4 which is more stable than NO2 since N2O4 does not contain any odd electron. The reaction is shown below:
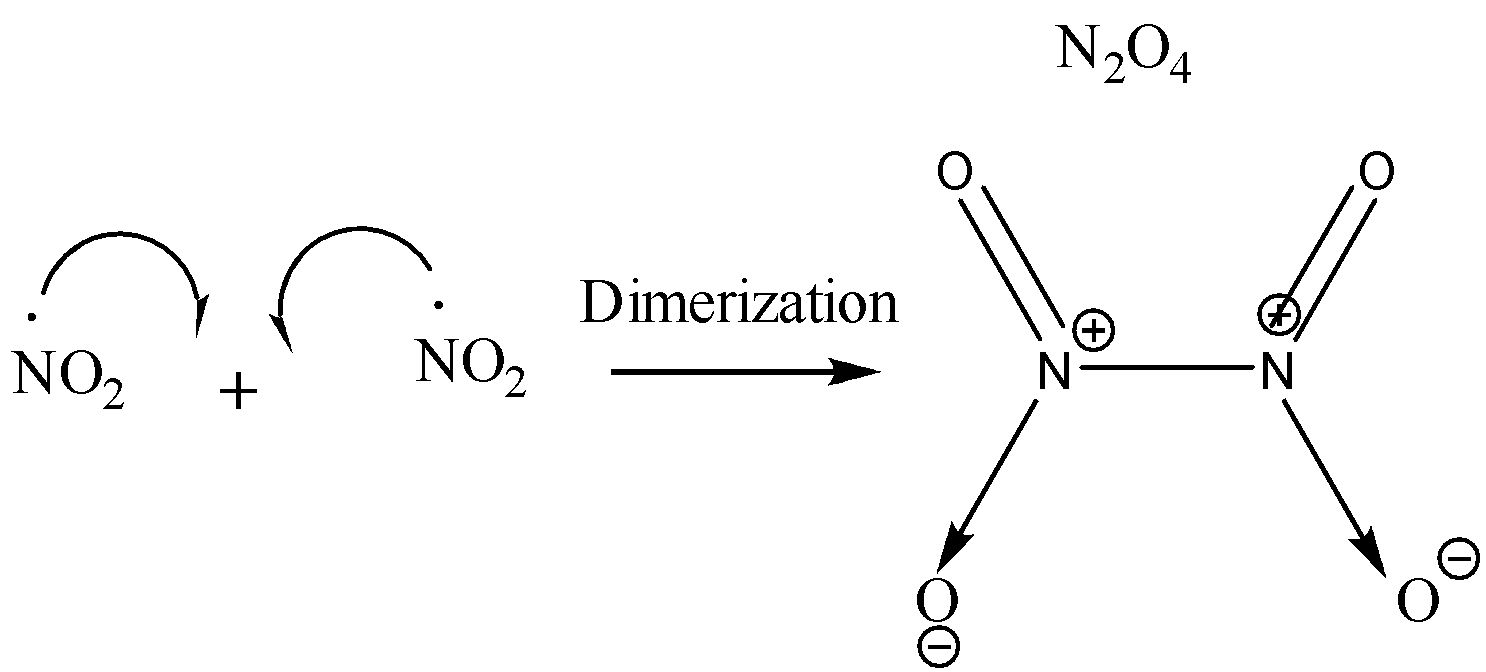
The presence of odd electrons in NO2 has been confirmed by experimental analysis too. Since NO2 has an odd electron, therefore it is weakly paramagnetic in nature but on dimerization, it loses its Para magnetism and becomes diamagnetic N2O4.
Therefore the dimerization of NO2 is due to the presence of an odd electron.
Note: For the formation of a dative bond, one atom needs to donate its electron pair to another atom. In this process, the donor atom acquires a positive charge since it donates its electrons and the accepting atom acquires a negative charge since it accepts the electron pair.
