Question
Question: Why does more heat transfer take place in a reversible process than in an irreversible process?...
Why does more heat transfer take place in a reversible process than in an irreversible process?
Solution
To answer this question, one must know about the laws of thermodynamics, firstly here we will use second law of thermodynamics for reversible process to find the ratio of heat transfer to temperature i.e., entropy and then for irreversible process and after comparing the equation we can see that why does more heat transfer take place in a reversible process than in an irreversible process.
Complete Step By Step Answer:
For reversible process:
A reversible process is one that takes an infinitesimally long time to complete. A reversible heat flow can be considered as the most efficient way to carry out a specific thermodynamic event, as it wastes no thermal energy. In reality, such a procedure does not exist, but we can get close.
From second law of thermodynamics we have,
dS=Tδqrev
Where,
dS is the change in entropy,
δq is the heat transfer and
T is the temperature.
Because entropy is a state function, the change in entropy must be expressed as a closed integral, as follows:
∮dS=∮Tδqrev=0
This means that if a process starts in one place and concludes in the same place, regardless of the path travelled, the entropy change must be zero.
As a result, in the second part of its course, the process must have perfectly reversed itself, resulting in zero entropy change.
This can now be expressed as the area under the curve of a T vs S graph for a nontrivial path where Δs=0
ΔS=1∫2T1δqrev
For irreversible process:
However, an irreversible process has expended some energy in the process. For example, one can transmit heat, pause for a minute, and then resume.
The delay allows heat to flow into another system in such a way that regaining it through the tiny reverse process is impossible.
A step-ladder process is a frequent approach to illustrate this:
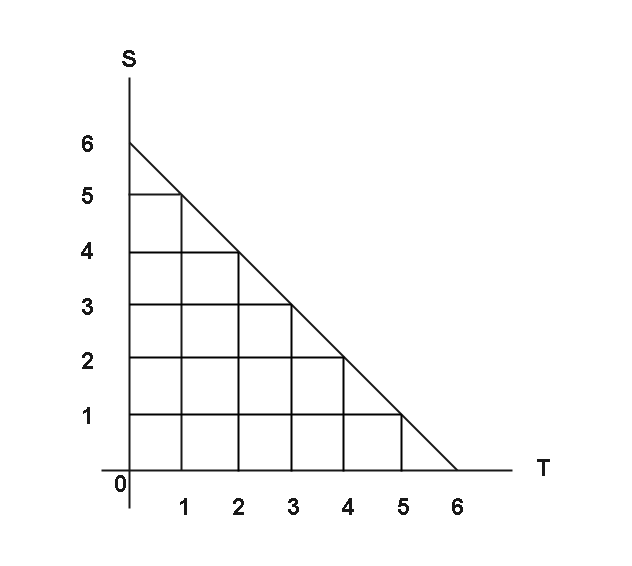
It's like using rectangles to approximate (and underestimate) the area under the curve for the T vs. S graph above. The area of these rectangles would provide the irreversible heat flow:
ΔS>T1(q1+12+q3)
Where,
qrev>q1+12+q3
The reason why ΔS cannot be associated to Tqirr by an equality is that if it were, even if one could design a path that leads to a closed integral, some heat would be wasted, which would violate the equality. That is to say,
∮dS=0 if dS=Tδqirr .
And also note that the different way to write the above equation is that:
qrev=qirr+qlost
Or
[ΔSrev=Tqrev]=Tqirr+Tqlost
After generalizing we get, since, Tqlost>0 , we write
ΔS⩾Tq
Or for infinitesimal changes,
dS⩾Tδq
Concluding, we got Tδqrev⩾Tδq
From the above explanation we can conclude that,
Hence, we can see that the reversible process is the infinitesimally long process which requires continuous allowance of energy to continue the effect whereas the irreversible process does not require a long-time energy source.
Therefore, heat transfer in a reversible process takes more time than the irreversible process.
Note:
The second law of thermodynamics asserts that the entropy of an isolated system can never decrease over time and remains constant if and only if all processes in nature are reversible. Isolated systems spontaneously evolve towards thermodynamic equilibrium, the condition with the highest entropy.
In ideal instances, where the system is in thermodynamic equilibrium, or we can say is performing a (fictive) reversible process in nature, the entropy of the overall surroundings and the system can remain constant. The overall entropy of the system and its surroundings grows in all processes that occur, including spontaneous activities, and the process is irreversible in nature and in the thermodynamic sense. The irreversibility of natural processes, as well as the asymmetry between past and future, are explained by entropy increases.
