Question
Question: Why does glucose not undergo Schiff’s base?...
Why does glucose not undergo Schiff’s base?
Solution
Schiff’s base: Aldehydes and ketones on treatment with primary aliphatic or aromatic amines in the presence of a trace of acid yields a Schiff’s base.
Complete step by step answer:
It contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group-but, not hydrogen. They have the general formula R1R2C=NR3
where R is an organic side chain. Hence, it is an amine.
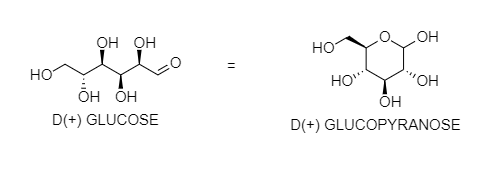
Glucose does not react with Schiff's reagent and 2,4 DNP reagent although it has an aldehydic group. You can see that OH at 5 - carbon reacts with the aldehyde group at 1 carbon to form hemiacetal in a cyclic form. After the internal cyclization, it forms either α- anomer or β-anomer. In these forms, a free aldehyde group is not present. So, it does not give the reaction of the aldehydic group.
Uses of Schiff’s base:
Schiff bases are common enzymatic intermediates
They are common ligands in coordination chemistry.
They were one of the first ligands used for asymmetric catalysis.
Schiff’s reagent is rosaniline hydrochloride (whose pink color is decolorized by passing SO2 gas). This reagent is generally used to detect the aldehydic group.
Note: The possibility to make a mistake is that Schiff’s base is a weak base, not a strong base, so it cannot get broken by this reagent as it is quite stable.
