Question
Question: Why does aluminium trichloride, \(A{l_2}C{l_6}\), sublime at the relatively low temperature of \({18...
Why does aluminium trichloride, Al2Cl6, sublime at the relatively low temperature of 180∘C?
1.The intermolecular forces between Al2Cl6 molecules are weak
2.The coordinate bonds between aluminum and chlorine are weak
3.The covalent bonds between aluminum and chlorine are weak
A.1,2 and 3 are correct
B.1 and 2 only are correct
C.2 and 3 only are correct
D.1 only is correct
Solution
We know that intermolecular forces are electrostatic in nature and include Vander Waals forces and hydrogen bonds. Molecules in liquids are bonded to other molecules by intermolecular interactions that are weaker than the intramolecular interactions that hold the atoms together within molecules and polyatomic ions. The three major types of intermolecular interactions are,
-Dipole-dipole interactions
-London dispersion forces
-Hydrogen bonds
Complete step by step answer:
We know that sublimation takes place when a substance changes from a solid into a gas. Increase in temperature causes the kinetic energy of particles to increase. This permits the particles to overcome the intermolecular forces and become mobile. Low pressure increases the kinetic energy of particles. As the particles escape the solid and disperse as a gas, sublimation takes place.
We have to remember that sublimation depends on the intermolecular force of attraction.
Sublimation and intermolecular force of attraction are directly proportional to each other.
Stronger the intermolecular force of attraction, higher will be the sublimation temperature.
The structure of aluminium chloride is,
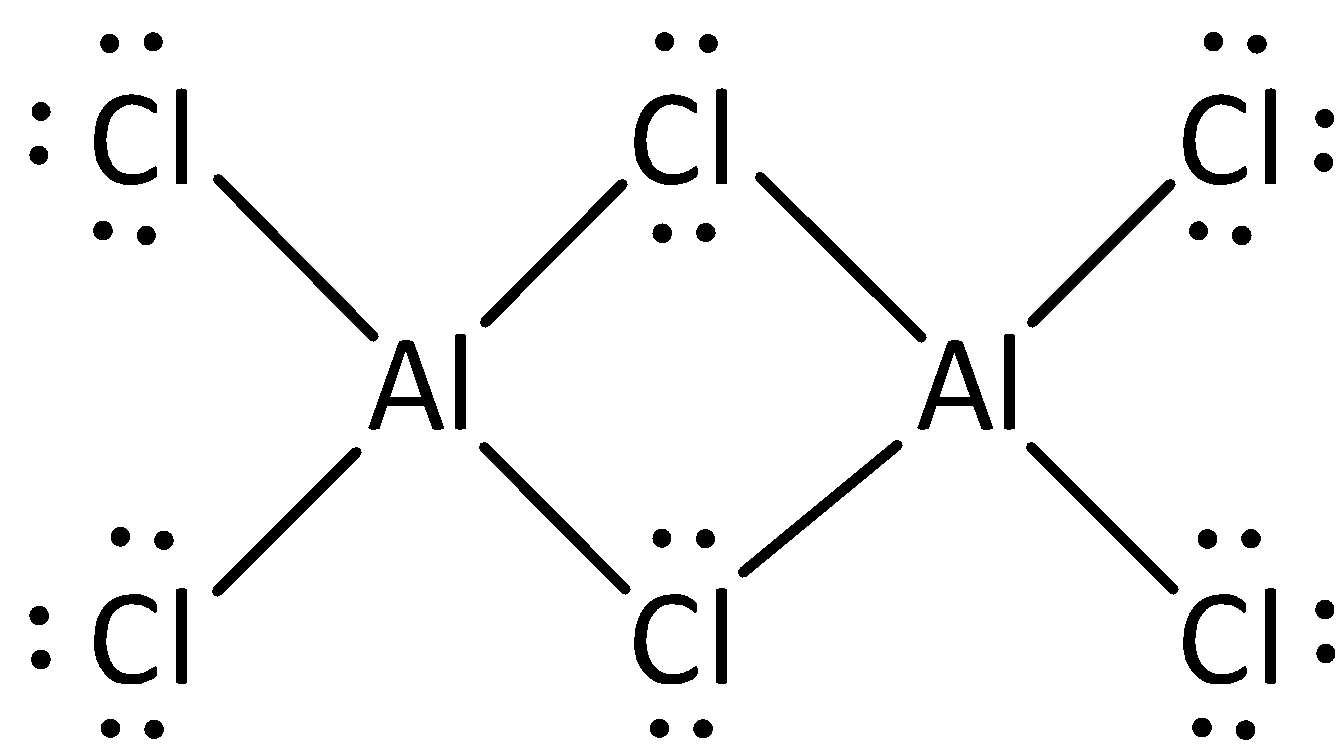
Aluminium chloride Al2Cl6 is a dimer and it shows weaker intermolecular forces.
Because of the presence of weaker intermolecular forces, Aluminium chloride sublimes at low temperature of 180∘C.
Only the statement (1) is correct. Therefore, Option (D) is correct.
Note:
We know that electronegativity increases across the period; aluminum and chlorine do not differ enough in electronegativity to form a simple ionic bond. The structure of aluminum chloride varies with respect to temperature. At room temperature, the aluminum is 6-coordinated. Each aluminum atom is surrounded by 6 chlorine atoms. The structure is an ionic lattice, but it has a lot of covalent character. At room temperature, solid aluminum chloride does not conduct electricity because the ions are not free to move. Molten aluminum chloride is also nonconductive as it has lost its ionic character.
