Question
Question: Why does acetylation of \[-N{H_2}\] group of aniline reduce its activity?...
Why does acetylation of −NH2 group of aniline reduce its activity?
Solution
The electron-donating and electron-withdrawing power of the attached functional group is responsible for the activation and deactivation of the phenyl ring. The electron-donating functional group enriches the electron density of the phenyl ring and the electron-withdrawing functional group pulls the electron density of the phenyl group.
Complete answer:
Let us start with aniline and the reason for its increased activity of the phenyl group. In aniline, an amine −NH2 functional group is attached to a phenyl ring. The presence of lone pairs on N undergoes delocalization in the phenyl ring by a phenomenon called resonance. It can be shown as:
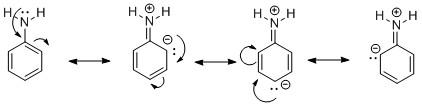
Due to the movement of electron pairs in the phenyl ring, the ring becomes very reactive towards electron-deficient electrophiles. Thus the amine group increases the activity of the phenyl group.
But when the amine group undergoes acetylation, the activity of the phenyl group reduces. We know that the acetyl group contains a carbonyl group which is electron-deficient and attracts the lone pair of electrons present on N towards itself. As a result, the lone pair of N can no longer take part in resonance with the phenyl ring. It rather moves towards the more electron-deficient carbonyl group of acetyl. This is shown as:
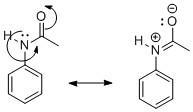
Due to the delocalization of electron pairs in the carbonyl functional group, the nucleophilicity of the phenyl group decreases. As a result, its reactivity decreases. That is why acetylation of the −NH2 group of aniline reduces its activity.
Note:
If the alkylation acetanilide is performed, then an O-alkylated side product is generated which clearly indicates the presence of O− nucleophile in acetanilide. Other electron-donating groups are OH, OMe, Me etc which increase the activity of phenyl ring while NO2, COOH, CN etc decreases the activity of phenyl ring.
