Question
Question: Why do some people call \( {({H_3}C)_4}C \) as neopentane instead of dimethyl propane?...
Why do some people call (H3C)4C as neopentane instead of dimethyl propane?
Solution
There are two types of names that are given to chemical compounds, especially organic compounds. These names are IUPAC names and common names. The IUPAC names are given according to the rules set for the nomenclature of organic compounds by the IUPAC (International Union of Pure and Applied Chemistry) while there are no strict rules to give the common names to the compounds.
Complete Step By Step Answer:
We should know that there are two types of names that are given to chemical compounds, especially organic compounds. One name is common name and the other name is IUPAC name. The IUPAC names are given according to the rules set for the nomenclature of organic compounds by the IUPAC (International Union of Pure and Applied Chemistry) while there are no strict rules to give the common names to the compounds.
First we will see the structure of dimethylpropane:
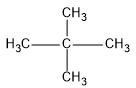
So, as the name suggests, there is a propane chain (longest carbon chain) and two methyl groups are present at the second position of the propane chain.
This compound is also known as neopentane because neopentane is the common name for this compound. This common name is given because dimethylpropane is an isomer of pentane.
Note:
We should know that neopentane or dimethyl propane is a double branched alkane. It contains five carbon atoms in its structure. At room temperature and pressure, it is a flammable gas which can be condensed into a volatile liquid when the temperature is lower down (in winters or when kept in an ice bath).
