Question
Question: Why do \( SN2 \) reactions prefer aprotic solvents?...
Why do SN2 reactions prefer aprotic solvents?
Solution
Aprotic solvents are neutral solvents. They increase the rate of SN2 reaction. The SN2 reaction is a bimolecular nucleophilic substitution reaction. The rate of the reaction depends on the concentration of both the nucleophile and the molecule undergoing attack.
Complete answer:
The SN2 reaction is a nucleophilic substitution reaction where a bond is broken and another is formed simultaneously. The term ‘ SN2 ’ means Substitution nucleophilic bimolecular and so this type of reaction is also referred to as bimolecular nucleophilic substitution reaction. In this reaction, the rate of the reaction is determined by two reacting species.
During the mechanism of SN2 reactions, some of the factors are considered, such as the presence of a strong anionic nucleophile, stability of the anion, and presence of aprotic solvents. The SN2 reactions usually prefer aprotic solvents.
The aprotic solvents are neutral and they neither donate nor accept any proton.
A solvent is required in these reactions to facilitate the collision between the nucleophile and the electrophile.
Let us take an example of the aprotic solvent DMSO (Dimethyl sulfoxide):
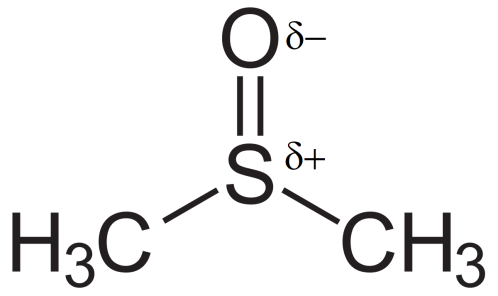
We see here that this solvent has a δ− and δ+ charge. Due to this δ− it becomes easy for the solvent to solvate the counter ion thereby releasing the nucleophile for attack. Also as the δ+ is crowded it will not be able to solvate the nucleophile. Thus the nucleophile is free to attack the substrate. Whereas if there is a protic solvent present, it will solvate both the counter ion as well as the nucleophile and so the nucleophile is not free to attack.
Note:
The SN2 reactions are the single step reactions. There is no intermediate formation. This reaction does not produce any carbocation too. This reaction product of this reaction is inversion of the reaction and it is called Walden's inversion.
