Question
Question: Why do ionic compounds have a high melting point? A. a considerable amount of energy is required t...
Why do ionic compounds have a high melting point?
A. a considerable amount of energy is required to break the strong inter-ionic attraction.
B. Ionic compounds have covalent bonds
C. Both A and B
D. None of the above
Solution
Ionic compounds are formed by two oppositely charged ions which are held together by electrostatic forces known as ionic bonds. In ionic compounds one metal ion is present and one non-metal ion is present.
Complete step by step answer:
The ionic compounds are formed by reacting a metal with a non-metal. During the reaction, metal donates its electrons to its neighboring element to fulfill its orbit while non-metal accepts electrons from its neighboring element to complete its orbit.
The metal ion and the non-metal ion are held together by each other through a strong electrostatic force of attraction.
Example: NaCl structure
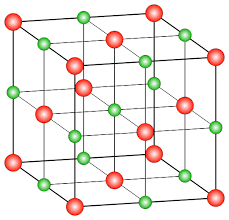
In the NaCl crystalline structure, the sodium ion attracts the chloride ion and vice-versa which leads to the formation of a three-dimensional structure of sodium chloride. The force of attraction between the ions holds the ions in the crystal structure. This results in a large structure of ions. As ions are held tightly by a strong force of attraction in the large structure, they require a huge amount of energy to break the bond.
This is why ionic compounds have high melting and boiling point.
Therefore, the correct option is A.
Note:
The ionic compound structure depends on the size of the metal cation and non-metal anion. The covalent compounds are formed of two non-metals by sharing of electrons and are held together by covalent bonds.
