Question
Question: Why dipole moment of \[{\text{P}}{{\text{F}}_2}{\text{C}}{{\text{l}}_3}\] is zero but that of \[{\te...
Why dipole moment of PF2Cl3 is zero but that of PF3Cl2 is non-zero?
Solution
PF3Cl2 and PF2Cl3 are the type of phosphorous Penta halide. The hybridization of these compounds is sp3d. And the geometry is trigonal bipyramidal. Where the less electronegative atoms are in equatorial position and more electronegative atoms are in axial position.
Complete step by step answer:
Now in the structures of PF3Cl2 and PF2Cl3 the axial positions are occupied by two fluorine atoms where the dipole moments of each P - F bonds nullify with each other. In case of equatorial bonds of PF2Cl3 all three P - Cl bond dipoles nullify with each other. Therefore in PF2Cl3 all the bond dipoles get cancel out and shows zero dipole moment.
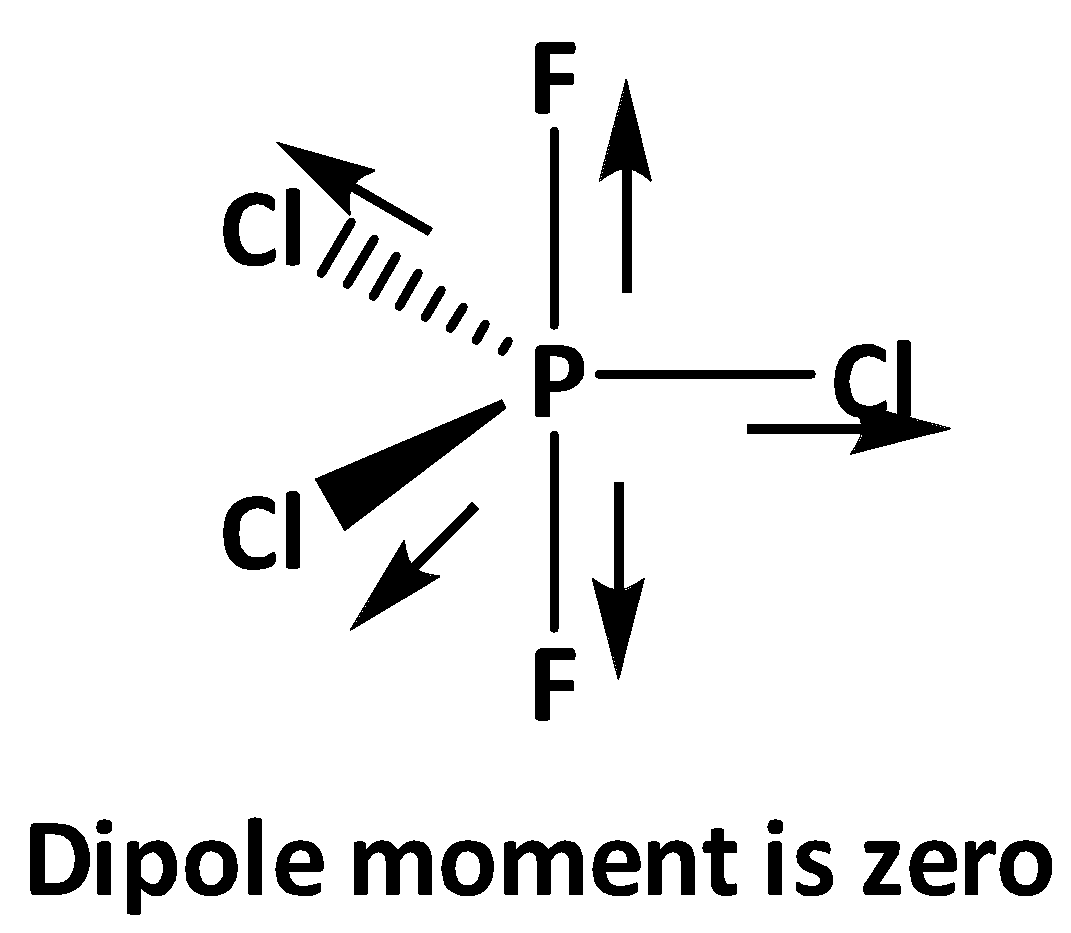
On the other hand, for PF3Cl2 in the equatorial bonds two chlorine and one fluorine is present, as electronegativity of fluorine is greater than chlorine , the overall net dipole moment is to the direction of the P - F equatorial bond.
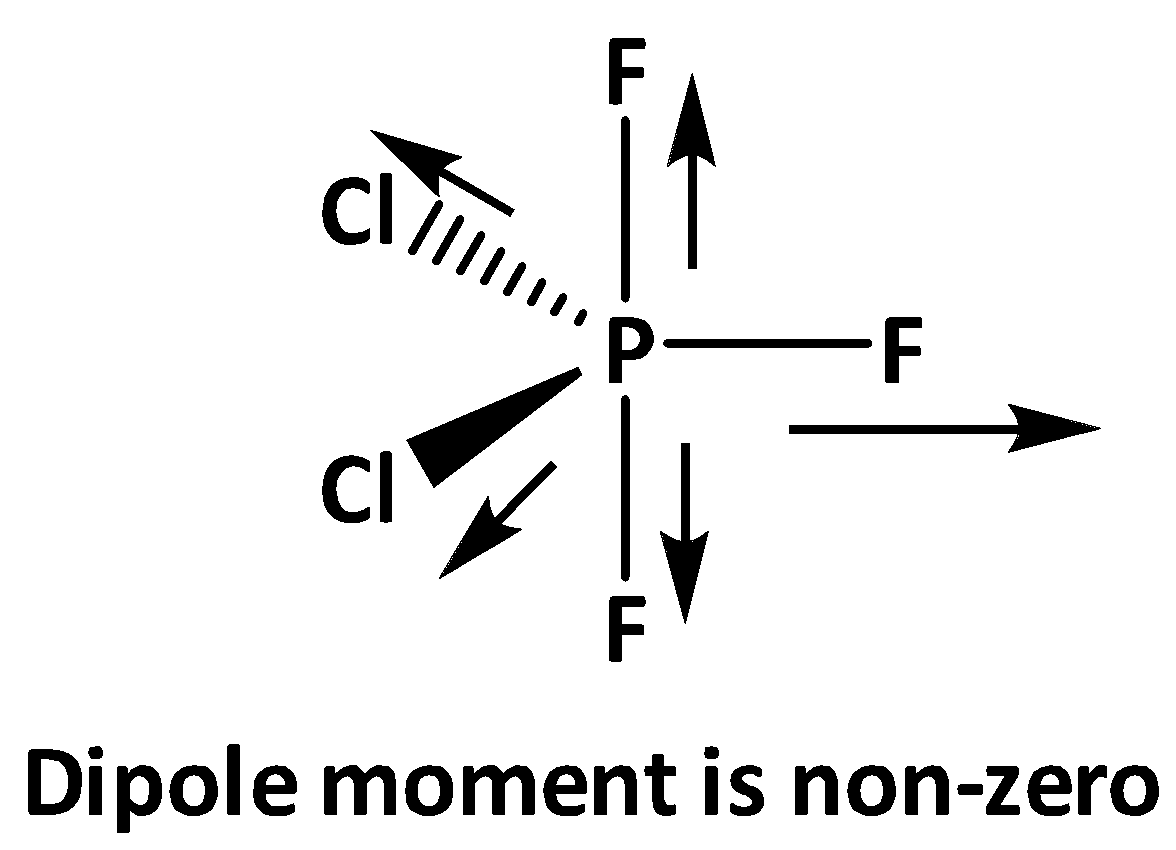
That is why PF3Cl2 shows a non-zero dipole moment. On the other hand, PF3Cl2has no dipole moment.
Note:
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes) suitable for pairing of electrons to form chemical bonds .Hybrid orbitals are the combination of standard atomic orbitals resulting in the formation of new atomic orbitals.
