Question
Question: Why cyclooctatetraene is nonplanar....
Why cyclooctatetraene is nonplanar.
Solution
We must remember that the cyclooctatetraene is an unsaturated cyclooctane. The molecular formula is C8H8 . It is otherwise called annulene. This polyunsaturated hydrocarbon is a lackluster to light yellow combustible fluid at room temperature. In light of its stoichiometric relationship to benzene, Cyclooctatetraene has been the subject of much examination and some discussion. In contrast to benzene, cyclooctatetraene isn't aromatic despite the fact that its dianion. Its reactivity is normal for a common polyene.
Complete step by step answer:
We must remember that early examinations exhibited that Cyclooctatetraene didn't show the science of a sweet-smelling compound. Then, early electron diffraction tests inferred that the C-C bond distances were identical. However, X-beam diffraction information from H. S. Kaufman exhibited cyclooctatetraene to receive a few adaptations and to contain two unmistakable C–C bond distances. This outcome showed that Cyclooctatetraene is an annulene with fixed rotating single and twofold C-C bonds.
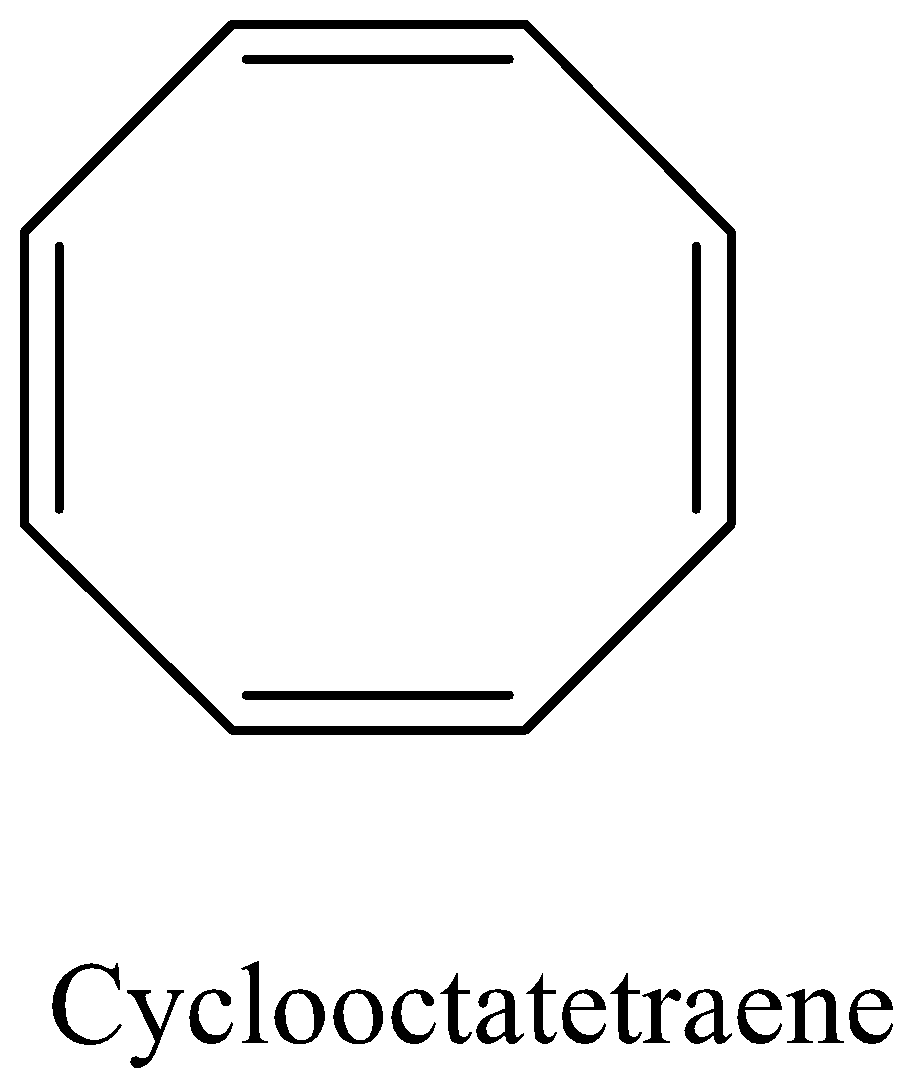
Any point strain from being planar is so immediately surpassed by the tremendous hop in steadiness that aromaticity brings that the atom will wind to frame a fragrant structure in a matter of seconds. In the event that cyclooctatetraene were planar, it would be an antiaromatic compound as indicated by Hückel's standard, since it has 8 pi-electrons
Note:
We must know that the pi bonds in Cyclooctatetraene respond as normal for olefins, as opposed to as sweet-smelling ring frameworks. Mono-and polyepoxides can be produced by response of Cyclooctatetraene with peroxy acids or with dimethyldioxirane. Different other expansion responses are additionally known. Moreover, polyacetylene can be orchestrated through the ring-opening polymerization of cyclooctatetraene. Cyclooctatetraene itself—and furthermore analogs with side-chains—have been utilized as metal ligands and in sandwich mixes. Cyclooctatetraene additionally goes through revamp responses to shape fragrant ring frameworks. For example, oxidation with watery mercury (II) sulfate structures phenyl acetaldehyde and photochemical revamp of its mono-epoxide structures benzofuran.
