Question
Question: Why \[C{H_4}\] cannot adopt square planar geometry?...
Why CH4 cannot adopt square planar geometry?
Solution
Any central atom having four atoms attached to it, can arrange in two shapes, square planar and tetrahedral on the basis of repulsion between those atoms, or lone pair on the central atom and the atom. Methane is a molecule with a carbon central atom and four hydrogen atoms attached to it.
Complete step-by-step answer:
Carbon is an element that belongs to group 13 of periodic table and has atomic number 6.
The Ground state electronic configuration of carbon is 1s22s22px12py1.
Excited state electronic configuration of carbon is 1s22s12px12py12pz1.
As four vacant orbitals can occupy four hydrogen atoms and form sp3 hybridised molecule,
commonly known as methane. sp3 hybridised molecules are tetrahedral in geometry and so is methane.
__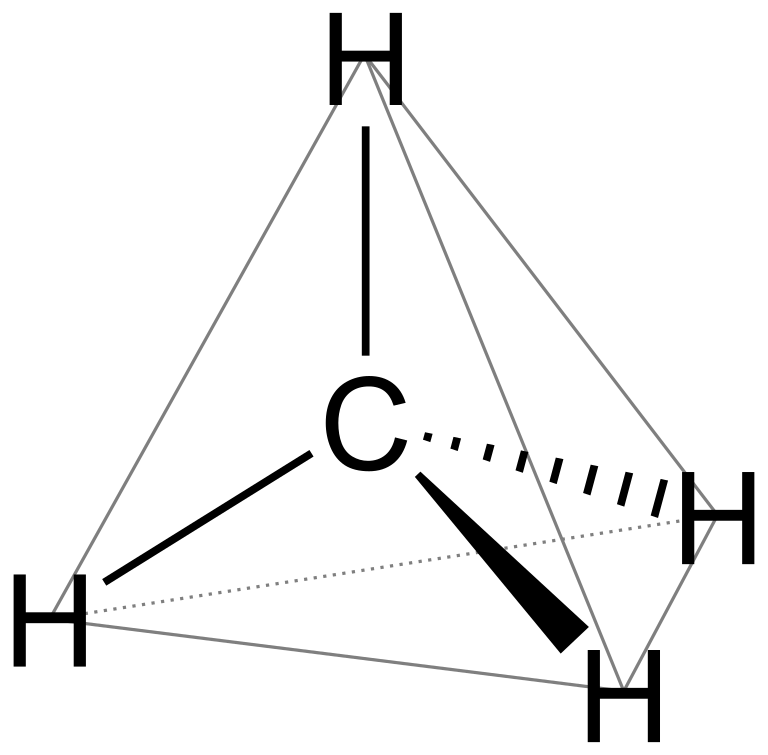
For square planar geometry, the hybridisation has to be dsp2. But in methane, there is no involvement of d-orbitals as it has less electrons. So, it cannot have square planar geometry.
As per valence shell electron pair repulsion theory (VSEPR), we can't determine the structure of any molecule with the help of its hybridisation.
Therefore, we say that CH4 cannot adopt square planar geometry.
Note: Methane molecule is tetrahedral with 109.5∘ bond angle (H-C-H), although the 2p orbitals are all at 900 to each other and four equal bond lengths with no dipole moment because the electronegativity difference between carbon and hydrogen is very less.
