Question
Question: Why are all P-F bonds in \(P{F_5}\) are not equivalent? A.\(P{F_5}\) has \(s{p^3}d\) hybridisation...
Why are all P-F bonds in PF5 are not equivalent?
A.PF5 has sp3d hybridisation, out of five P−F bonds three are equatorial which have different lengths.
B.PF5 has sp3 hybridisation, out of P−F bonds two are equatorial which have different lengths.
C.Out of five P−F bonds two are axial and three equatorial. All five bonds have different bond lengths.
D.PF5 is made up of two types of bond namely covalent and coordinate, hence are not equivalent.
Solution
Hint : VSEPR Theory (valence shell electron pair repulsion theory) plays an important role in predicting shape of a molecule. PF5 has 5 bond pairs of electrons and 0lone pairs of electrons which will be needed to know its shape. It’s hybridization is sp3d .
Complete step by step solution :
Bonds to a non ring atom with an angle of about 90∘ to the ring plane are termed axial.
Bonds to a non ring atom which make only a small angle compared with the plane of the ring are termed as equatorial bonds.
PF5 is phosphorus pentafluoride. It has a 5 bond pair and a 0 lone pair. VSEPR Theory is used to predict the geometry of individual molecules from the number of electron pairs surrounding the central atom. According to this theory if a compound has 5 bond pair and 0 lone pair. Then it will have trigonal bipyramidal geometry.
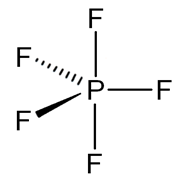
This kind of geometry has sp3d hybridization. This means PF5 has trigonal bipyramidal geometry in which 3 bonds are at equatorial position and two bonds are at axial position. Bonds at axial position are larger in length because they experience more repulsion by 3 equatorial P−F bonds, that is why all P−F bonds in PF5 are not equivalent.
So our answer to this question is option C that is Out of five P−F bonds two are axial and three equatorial. All five bonds have different bond lengths.
Additional information: To explain the concept of shapes of molecules clearly hybridization was introduced. It involves intermixing of two or more atomic orbitals of slightly different energies but of the same atom so that a redistribution of energy takes place between them resulting in the formation of an equal number of new orbitals which are called hybrid orbitals which will have the same energy, size and shape.
Note : PF5 is a neutral and non polar compound (compound having symmetric distribution of charge so that net charge is zero on compound) with 5 fluorine atoms attached to central phosphorus element and all these fluorine atoms are attached symmetrically around phosphorus metal.
