Question
Question: Who made the first law of Thermodynamics?...
Who made the first law of Thermodynamics?
Solution
Thermodynamics: The term Thermodynamics comprises heat, temperature and work. Basically it explains the relationship between these three parameters of a matter. It also explains how thermal energy can be converted into other forms of energy and vice-versa and its effects on matters.
Example of Thermodynamics: Thermosteel bottles to maintain the temperature of the liquid whether it's hot or cold. Melting ice cubes into liquids, Using microwaves for cooking purposes etc.
Complete step by step solution:
First Law of Thermodynamics: According to the first law of thermodynamics, in an isolated system, energy can neither be created nor it can be destroyed. This law is also known as “Energy Conservation Law”.This law was made by scientist Rudolf Clausius in 1850.
Additional information:
Other laws of Thermodynamics:
Second Law: According to the second law of thermodynamics the entropy of an isolated system always increases.
Third Law of Thermodynamics: According to the third law of thermodynamics, as the temperature of a system approaches absolute zero, the entropy of a system approaches a constant value.
According to energy conservation law, energy can neither be created nor it can be destroyed. Only it can be transferred from one form to another form of energy but the total amount of energy always remains the same.
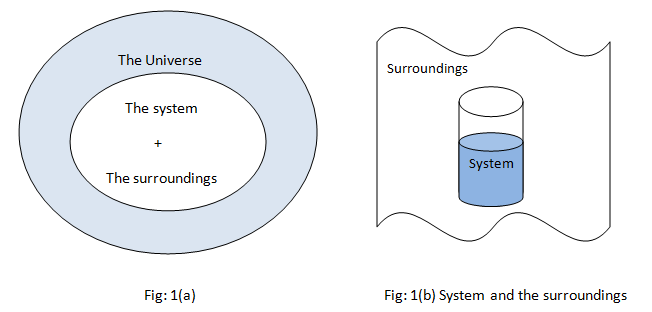
All the observations are made into the systems and the remaining part other than observations included into the surrounding. Various changes take place into the systems but it does not affect the entire universe, for practical purposes only surroundings can interact with the system as shown in fig 1(b).
Note: The second law is made by scientists Rudolf Clausius and William Thomson and the third law is made by scientist Walther Nernst during 1906−1912 while entropy can be explained as a measure of thermal energy per unit temperature of a system.
