Question
Question: Who is the first member of the ketone series?...
Who is the first member of the ketone series?
Solution
Ketones are organic compounds with the functional group CO, also known as carbonyl functional group, in the center of the compound. The general formula of ketone is RCOR’, unstable compounds are not included in the ketone series.
Complete answer:
The correct answer to this question is 2-propanone, which has the chemical formula of CH3COCH3 . It is also commonly known as acetone.
Ketones compounds are those compounds in which there is a carbonyl functional group, which is CO, attached to different alkyl groups.
The carbonyl group is in the centre of the compound and the alkyl groups are attached besides it. The general formula of ketone is RCOR’, a more detailed formula is shown below.
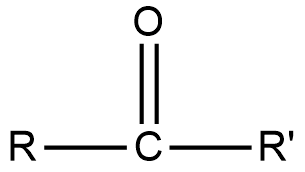
In this, the carbonyl group is at the centre, R represents the first alkyl group and R’ represents the second alkyl group. Now, to find the first member of the ketone series, we have to put the most basic alkyl group in R and R’.
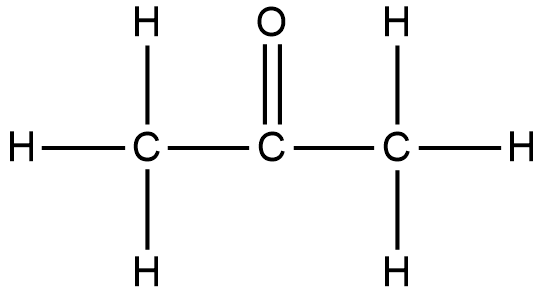
Thus, the most basic hydrocarbon is methane which has a formula of CH4 , thus putting it in place of R and R’, we get 2-propanone or propanone. Here, the CH4 becomes CH3 to satisfy the electron need of the carbonyl group.
The structure of 2-propanone or propanone is shown below.
Note:
Here, methanone or ethanone cannot exist as they cannot have a secondary carbonyl group, thus propanone is the first in the ketone series. The R and R’ can have any kind of alkyl group for the ketone series, but to find the first, basic hydrocarbons are put in their place.
