Question
Question: White phosphorus \({P_4}\) has the following characteristics. (This question has multiple correct ...
White phosphorus P4 has the following characteristics.
(This question has multiple correct options)
(A) 6 P-P single bonds
(B) 4 P-P single bonds
(C) 4 lone pair of electrons
(D) P-P-P angle of 60∘
Solution
Phosphorus is 15 group elements. Phosphorus is an essential constituent of animal and plant matter. It is present in bones as well as in living cells.
Complete step by step answer:
Phosphorus exists in several allotropic forms.
Example: White phosphorus, red phosphorus, black phosphorus, etc.
White phosphorus is in gaseous state and is a waxy solid consisting of reactive p4tetrahedron.
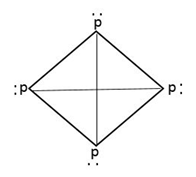
The structure of phosphorus shows 6 covalent bonds between P-atom and 4 lone pairs of electrons.
Therefore, from the above explanation, the correct option is [A] 6 p-p single bond and [C] 4 lone pair of electrons
Additional information:
White phosphorus is very reactive because p4 rings are held together by physical bonding but no chemical bonding.
These p4 rings are in unstable arrangement.
The red phosphorus consists of complex chain structure formed by opening of p4cage.
Black phosphorus has a layered structure and most thermodynamically stable form.
Phosphorus can be considered as light solid.
White phosphorus is very reactive and spontaneously ignites at 30∘C in moist air so usually it is stored under water. So that exposure to air can be prevented.
Red phosphorus is stable at room temperature but can be converted to white phosphorus by heat, sunlight or friction.
Note: White phosphorus crystallizes in a cubic system. Phosphorus atomic size is more so it can form a single bond with other phosphorus atoms. Hence it exists as tetra atomic molecules.
