Question
Question: White phosphorous (\({P_4}\)) has: A. four \(P - P\) single bonds B. four lone pair of electrons...
White phosphorous (P4) has:
A. four P−P single bonds
B. four lone pair of electrons
C. P−P−P angle of 60∘
D. six P−P single bonds
Solution
Four phosphorus atoms in white phosphorus (P4) molecule arranged in tetrahedral structure and form ring structure. White phosphorus is less stable and therefore, more reactive than the other solid phases under normal conditions because of angular strain in the P4 molecule. It readily catches the fire in the air.
Complete answer:
White phosphorous (P4) molecule has tetrahedral geometry. Electronic configuration of phosphorus atom: 1s2 2s2 2p6 3s2 3px1 3py1 3pz1 . Phosphorus atom has 5 valence electrons. Valency of phosphorus atom is 5 and each phosphorus atom forms 3 bonds in a (P4) molecule with other phosphorus atoms and one lone pair of electrons present on each phosphorus atom. Structure of white phosphorous (P4) molecules as:
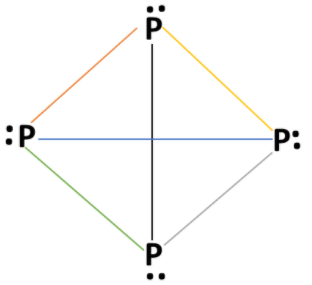
Each P atom has one lone pair of electrons and each covalent bond has two electrons of a bond pair. In a white phosphorus molecule, there are four lone pairs of electrons on four phosphorus atoms and six P−P single bonds are present. P−P−P bond angle is 60∘ in white phosphorus molecule.
Hence, correct answers are (B) and (C).
Additional Information: Tetrahedral arrangement of white phosphorus molecule results in ring strain and instability. White phosphorus (P4) molecule is not a planar molecule, it is a 3D molecule. White phosphorus is a translucent white waxy solid. It is insoluble in water and soluble in carbon disulphide.
Note: Valency of phosphorus atom is 5 and total four phosphorus atoms are present in a white phosphorus molecule. Total valence electrons in white phosphorus molecules will be 5 x 4 = 20 and lone pairs of electrons are 4 (each phosphorus atom has one lone pair of electrons).
