Question
Question: While separating a mixture of ortho and para- nitrophenol by steam distillation,name the isomer whic...
While separating a mixture of ortho and para- nitrophenol by steam distillation,name the isomer which will be steam volatile? Give reasons.
Solution
We should try checking the hydrogen bonding between the two compounds and that might give us an idea about their boiling point.So,in order to distinguish both of the isomers,we should try check the positions of the functional groups here.
Complete answer:
Ortho-nitrophenol shows intramolecular hydrogen bonding, which means bonding is present between it’s own molecule,between the phenol and nitro groups. Owing to this, when the given compound is subjected to steam distillation,the compound easily vaporizes out ,thus having a low boiling point and thus will be steam volatile.
Let’s try to understand by the diagram of the compound:
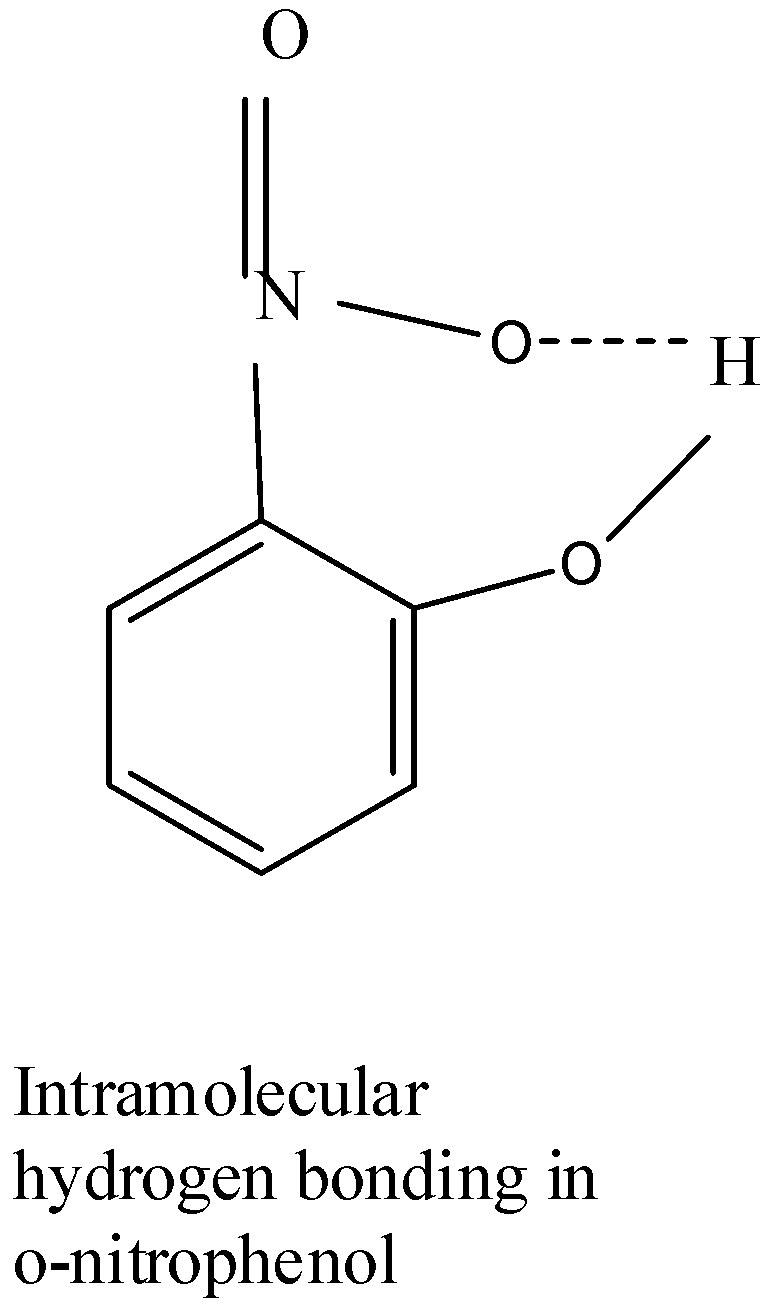
So, from the structure it is clear that it shows intramolecular hydrogen bonding.
The boiling point of ortho-nitrophenol is 216oC..
Now let us consider the compound para-nitrophenol
This structure ,shows intermolecular hydrogen bonding,which means that it can show bonding with other molecules, and thus it has a strong bond which when tried to separate by steam distillation, shows less volatility,as this type of bonding leads to molecular association.Therefore, this compound does not easily evaporates out, due to which it has higher boiling point and does not separate during the process.
Let us try to understand it’s structure:
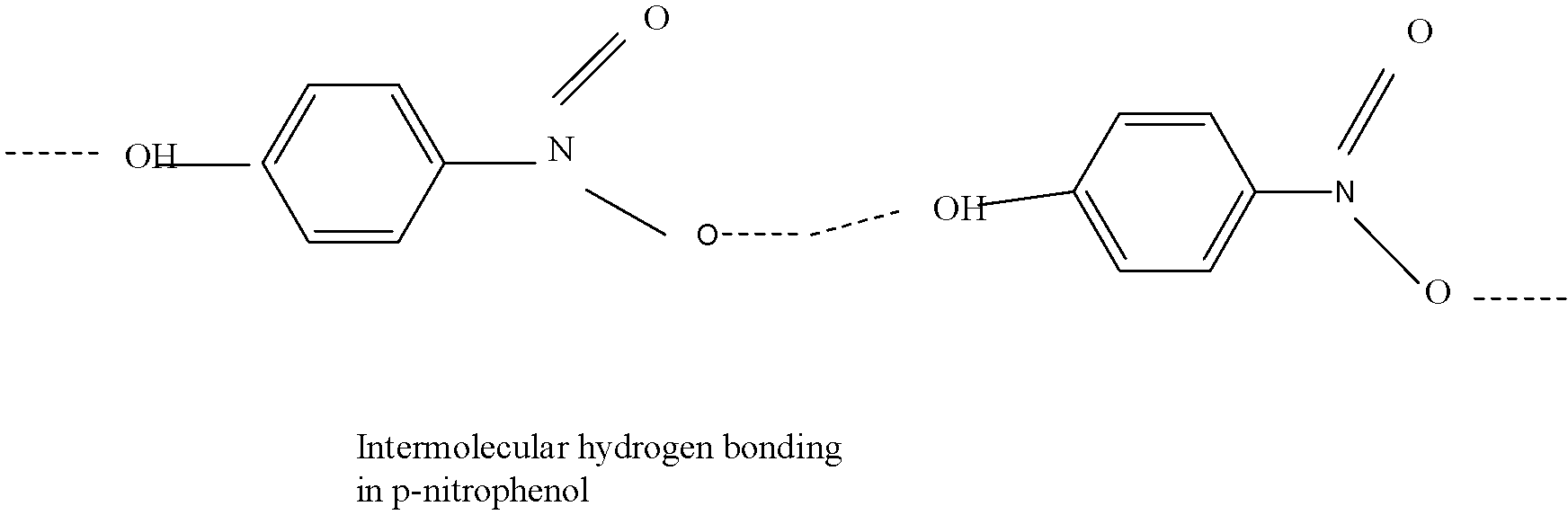
Now looking at the structure,it is prevalent that it shows bonding between its other molecules, and thus also shows a higher boiling point.
The boiling point of para nitrophenol is 279oC
Thus, we can infer that the ortho-nitrophenol is steam volatile then the para-nitrophenol owing to the above stated reasons.
Note:
We should remember that the steam volatile nature talks about the boiling point,which is checked by the type of bonding both the compounds show.The position of the isomers is of utmost importance.
