Question
Chemistry Question on Electrochemistry
While charging the lead storage battery
A
PbSO4 on anode is reduced to Pb
B
PbSO4 on cathode is reduced to Pb
C
PbSO4 on cathode is oxidized to Pb
D
PbSO4 on anode is oxidized to PbO2
Answer
PbSO4 on anode is oxidized to PbO2
Explanation
Solution
The cell reactions during charging of lead storage battery are
At anode
PbSO4(s)+2e−→Pb(s)+SO42−(aq)
At cathode
PbSO4(s)+2H2O(l)→PbO2(s)+SO42−(aq)+4H+(aq)+2e−
So, PbSO4 at the anode is reduced to Pb.
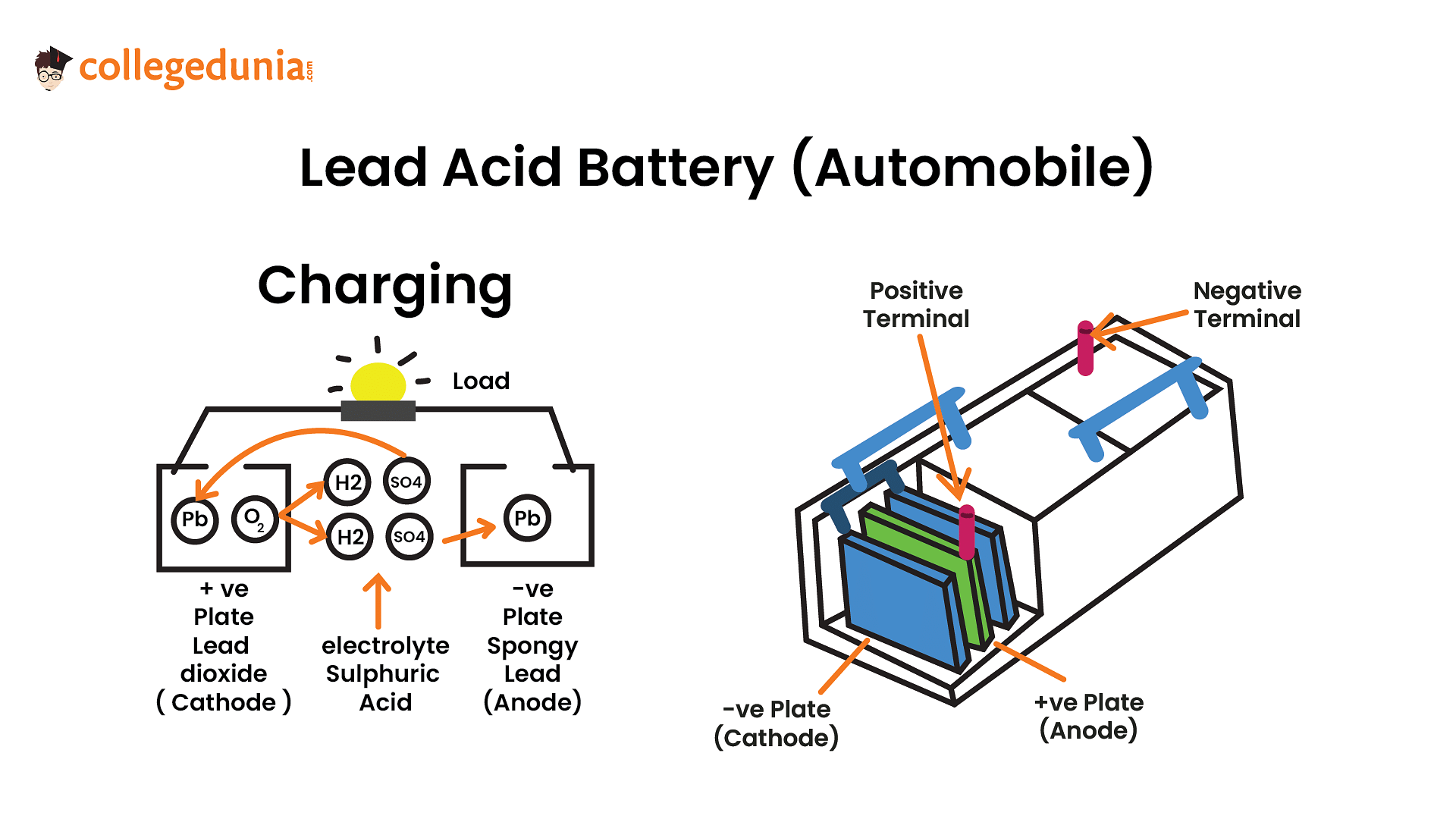
During charging, PbO2 is formed at the anode from PbSO4 (oxidation of Pb ion) and Pb is formed at the cathode from PbSO4 (reduction of Pb ion).
Therefore Options B and D are the correct answers.
Discover More From Chapter:Electrochemistry
