Question
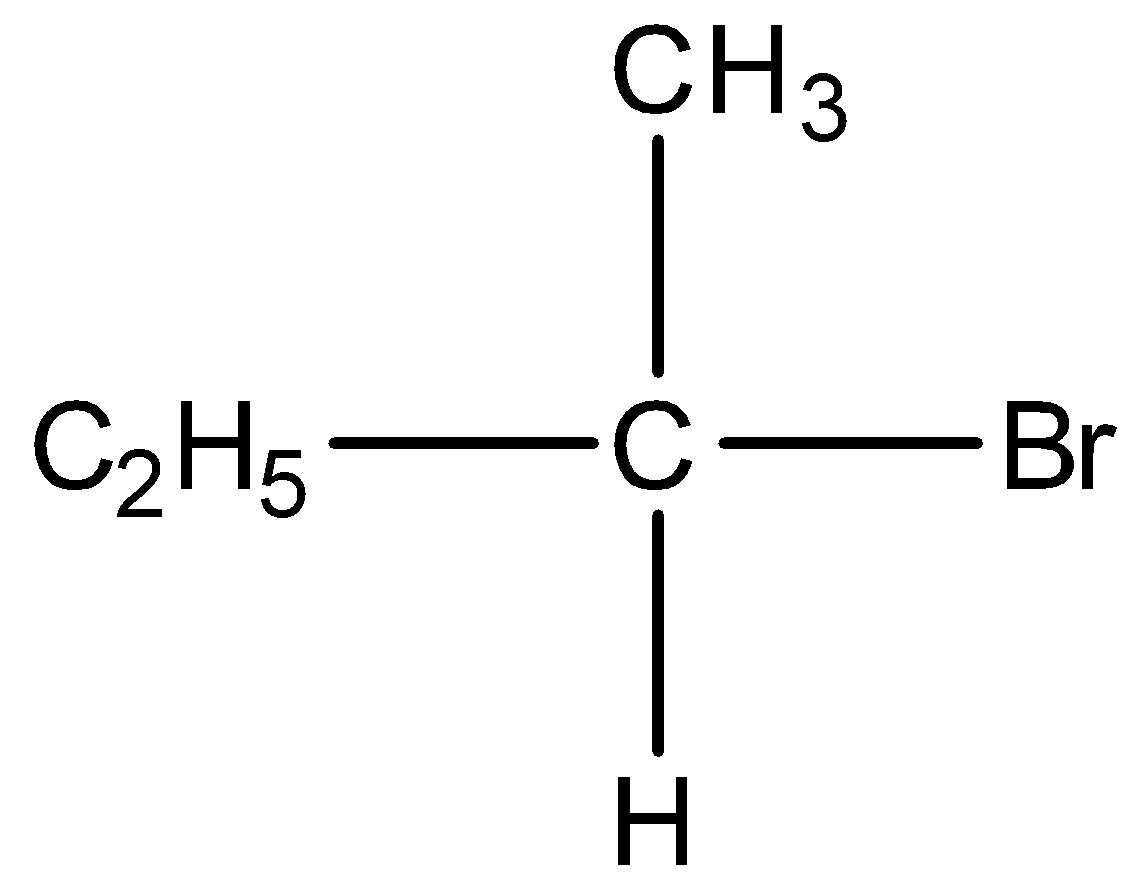
Question: Which will undergo the fastest SN2 substitution reaction when treated with NaOH? A) 
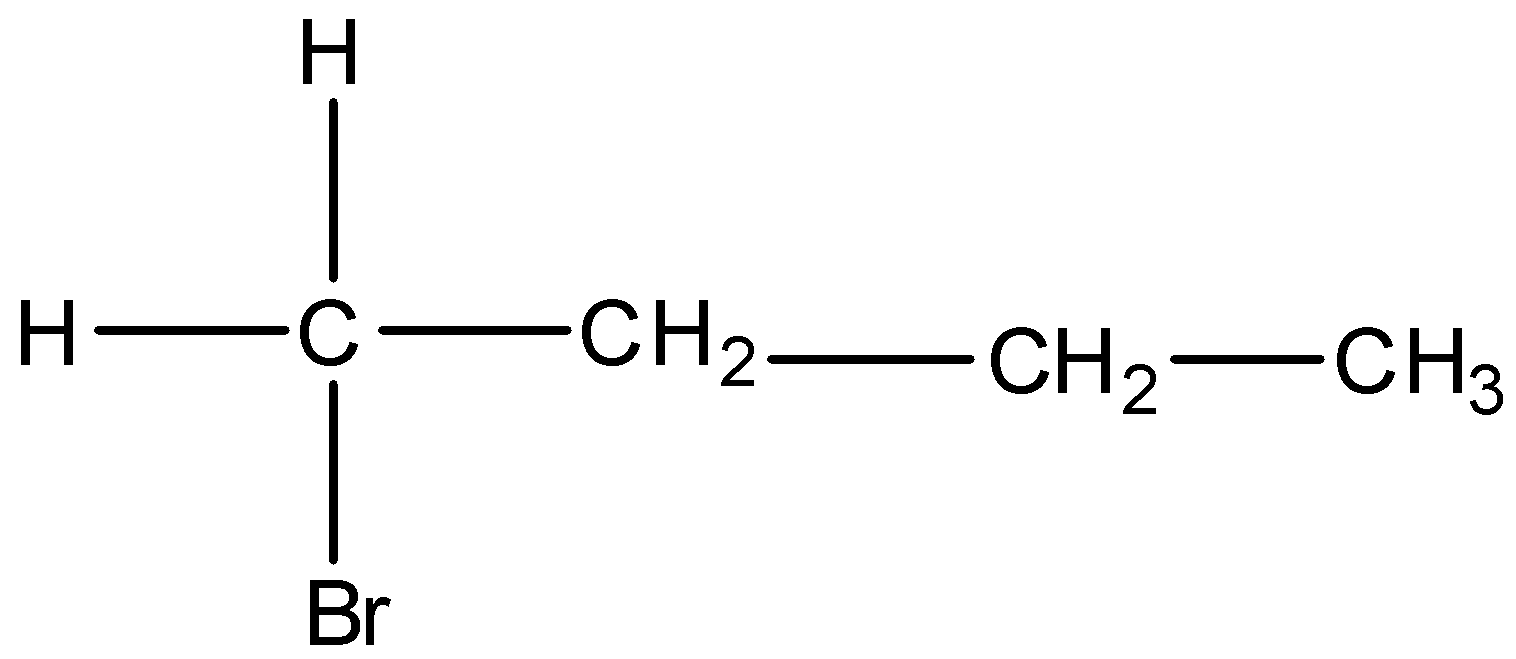
B)
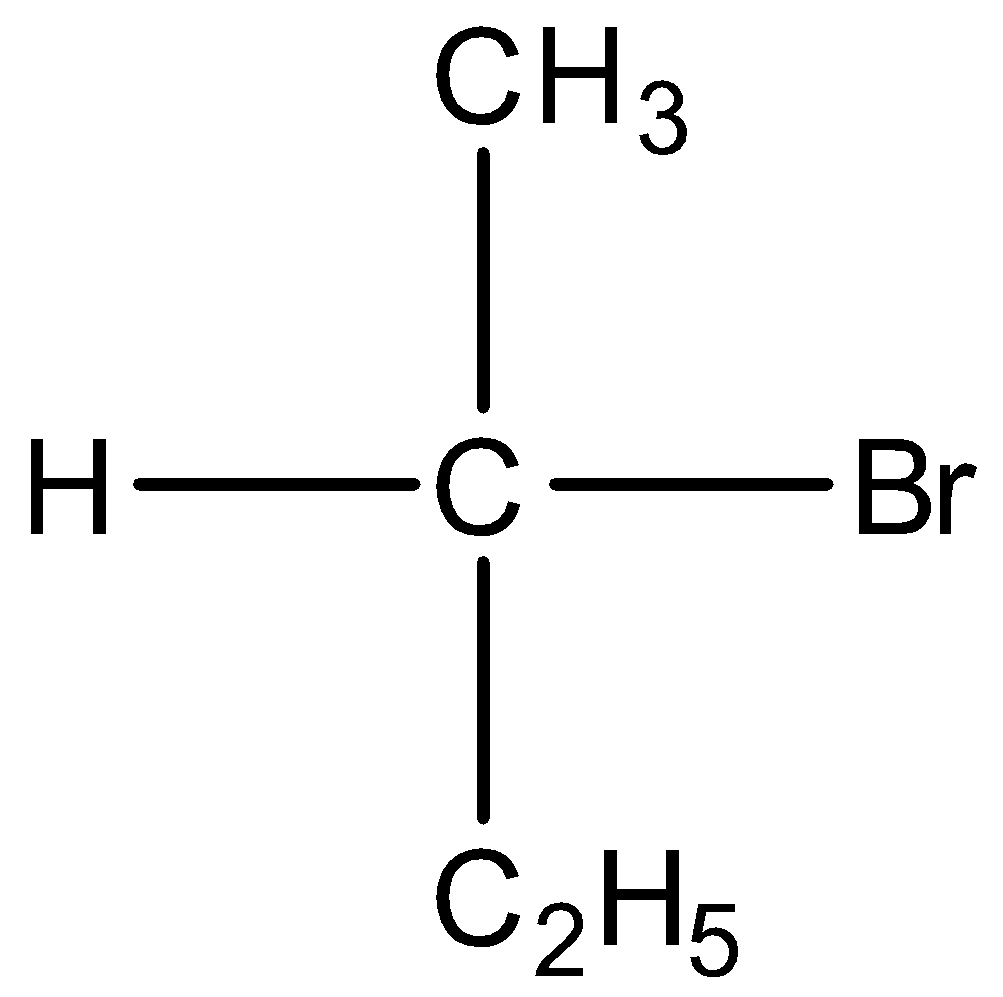
C)
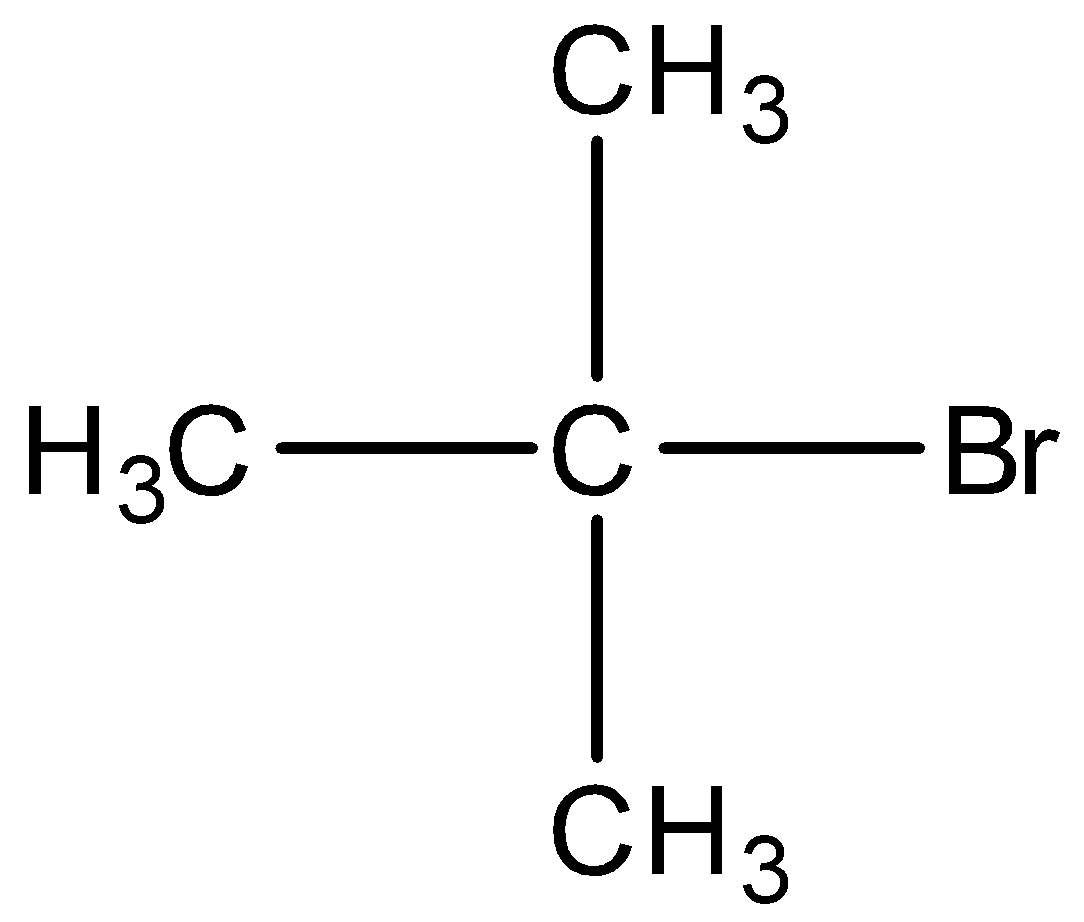
D) 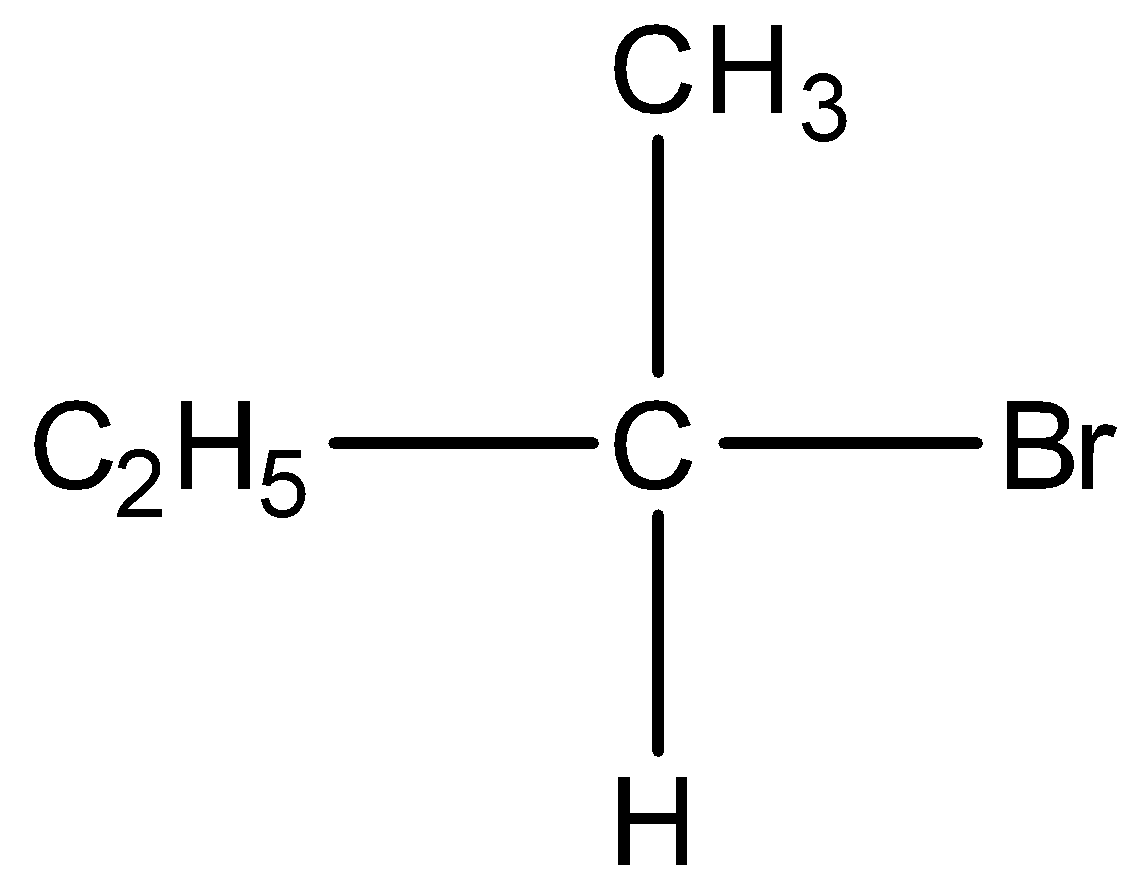
Solution
We must have to know that SN1 and SN2 reactions are nucleophilic substitution reactions. SN2 reactions are nucleophilic substitution reactions. It is a two step reaction which involves the formation of a transition state. The order of the reaction is two. There is no intermediate formation in SN2 reaction.
Complete answer:
Let us read about SN2 reactions in brief:
SN2 reactions are nucleophilic substitution reactions. It is a two step reaction which involves the formation of a transition state. The order of the reaction is two. There is no intermediate formation in SN2 reaction as there is SN1 reaction thus the order of stability for SN2 reaction is 1o>2o>3o whereas the order of stability for SN1 reaction is 3o>2o>1othat is the order of the stability of a carbocation.
Option A) this is a correct option as this option 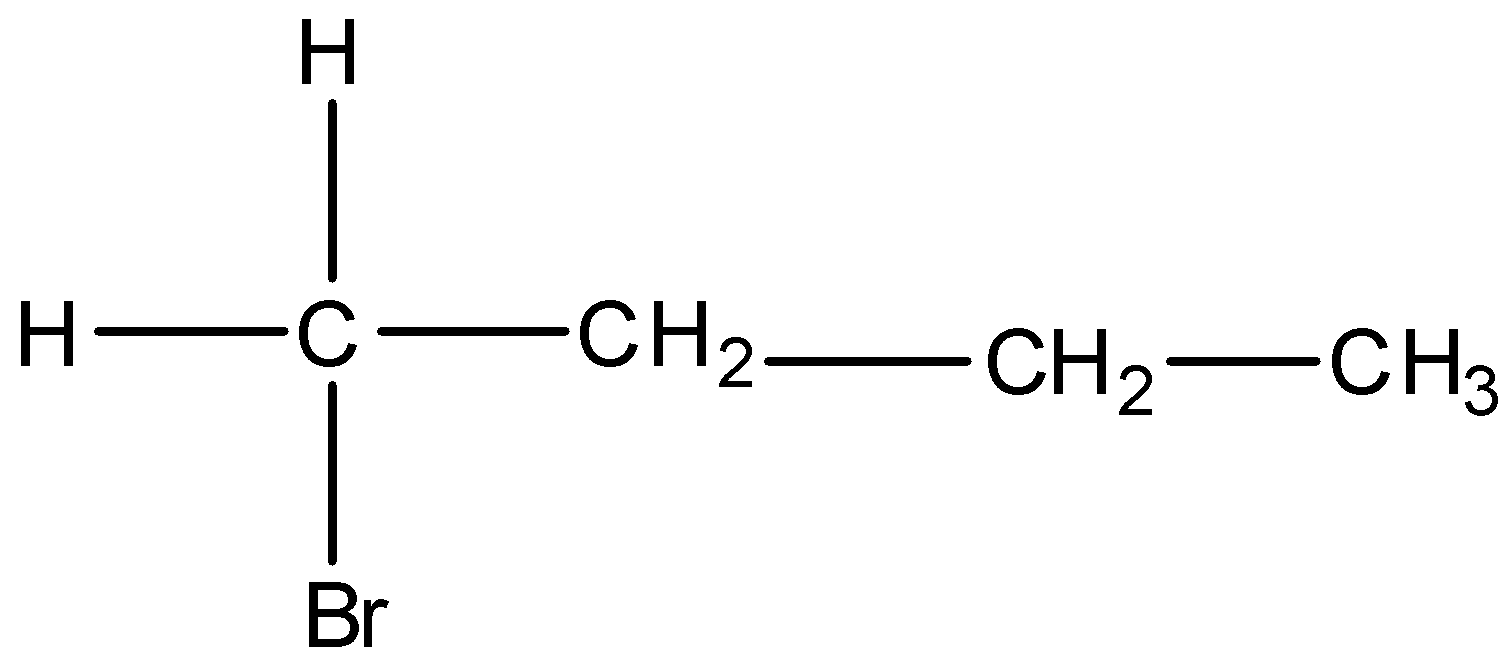
is a primary alkyl and the order of stability in SN2 reaction is primary, then secondary followed by tertiary therefore primary alkyl will show fastest SN2 nucleophilic reaction.
Option B) this is an incorrect option as it is a secondary alkyl 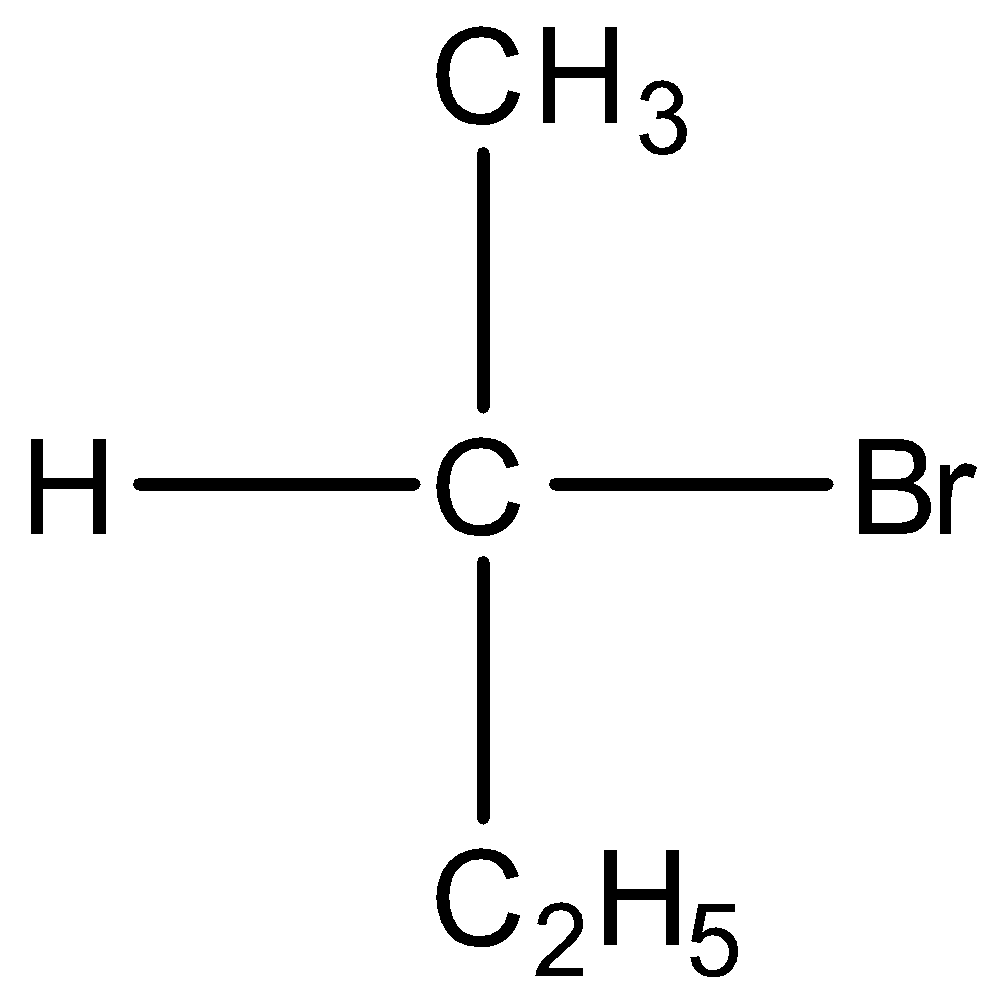
its stability is less than primary alkyl thus this will not show the fastest SN2 substitution.
Option C) this is an incorrect option as it is a tertiary alkyl 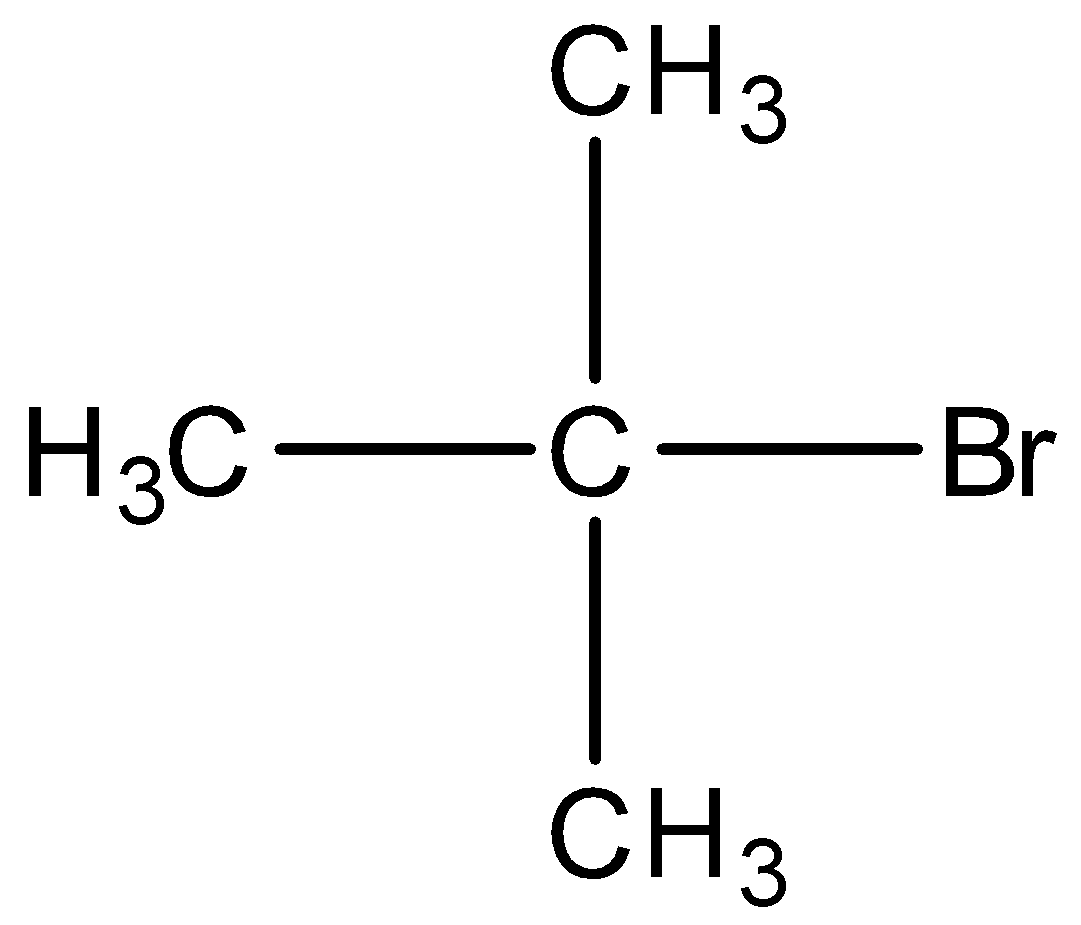
it will have the least stability as compared to primary and secondary, thus this option is not correct.
Option D) this is an incorrect option as it is a secondary alkyl 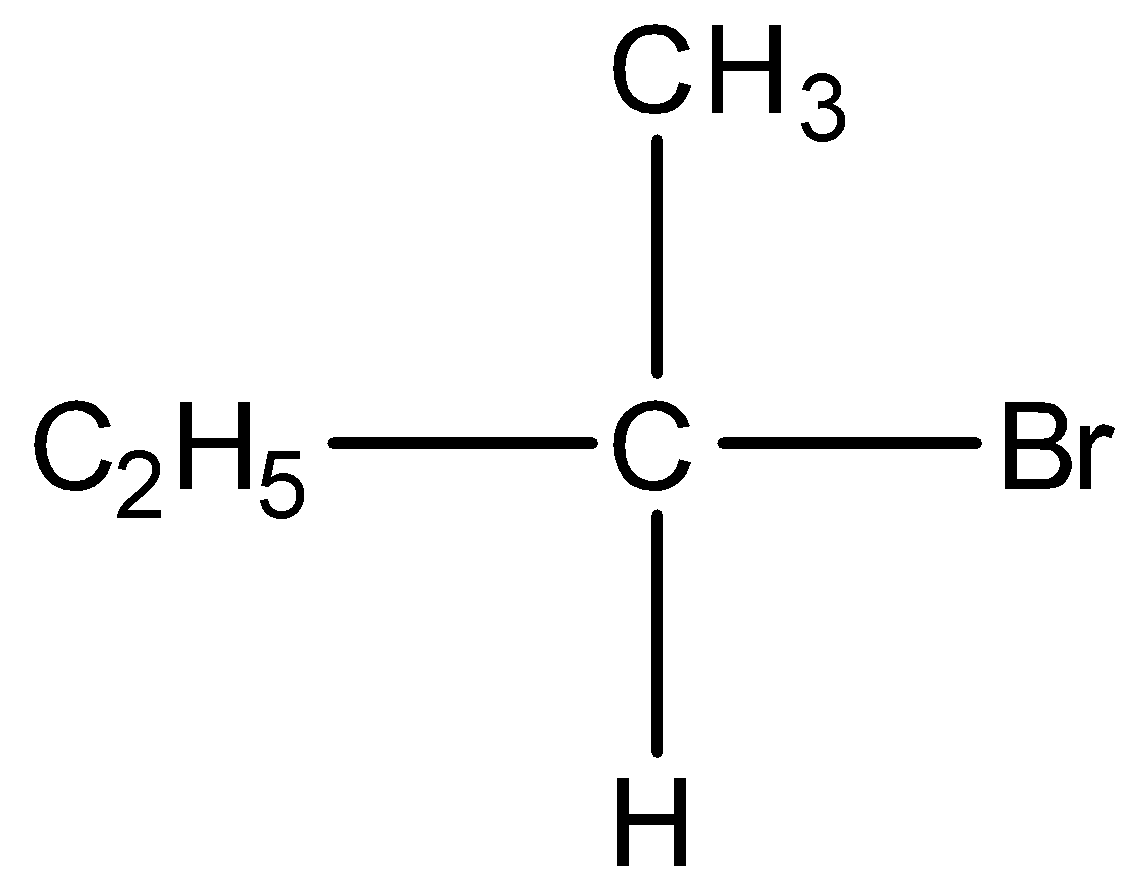
its stability is less than primary alkyl thus this will not show the fastest SN2 substitution.
Note:
We have to know that SN1 reactions are nucleophilic substitution reactions following the first order type. The order of stability depends upon the stability of a carbocation that is an intermediate. The rate determining step is always the slowest step.
