Question
Question: Which will form geometrical isomers. (A)  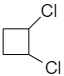
(B) 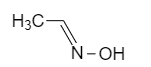
(C) 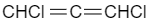
(D) Both a and b
Solution
Hint : The total number of ligands attached to a central metal atom or ion is called the coordination number of that ion.
Geometrical isomerism is not shown by complexes with coordination number 2 and 3, square planar complexes of the type MA4,MA3B,MAB3 and octahedral complexes of MA6,MA5B type.
Complete Step By Step Answer:
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. If the ligands occupy adjacent positions they are referred as cis-form and if the ligands occupies opposite positions they are referred as trans-form.
1,2-dichlorocyclobutane shows geometrical isomerism because it forms cis-1,2-dichlorocyclobutane and trans-1,2-dichlorocyclobutane as shown below

Oximes or N-hydroxy alkane mines also shows geometrical isomerism.
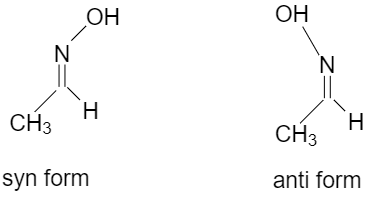
syn oxime is the one which has both hydrogen and hydroxyl groups attached to the C=N side and in anti oxime the hydrogen and hydroxyl group attached to the opposite of the C=N C=N side.
As the attached groups are present in different planes so 1,3-dichloroallene does not show geometrical isomerism.
So the correct answer for the question is option D. 1,2 dichlorocyclobutane and oxime will form geometrical isomers.
Note :
The mirror images compounds are non-superimposable on each other and do not possess the plane of symmetry. The optical isomers also possess the property of chirality.
The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They only differ in the direction in which they rotate the plane of polarised light.
