Question
Question: Which type of overlapping is present in \(S{O_3}\)? (A) \(p\pi - p\pi \) (B) \(p\pi - d\pi \) ...
Which type of overlapping is present in SO3?
(A) pπ−pπ
(B) pπ−dπ
(C) dπ−dπ
(D) Both A and B
Solution
In the formation of bonds in SO3 molecules, the d-orbitals of sulphur atoms take part. Oxygen atom forms a bond with a sulphur atom by its incompletely formed p-orbitals.
Complete step by step solution:
We know that according to Valence bond theory, it is required that overlapping between the atomic orbitals occurs in order to form a covalent bond.
-We know that a sigma (σ) bond forms when the overlap of an atom occurs between the nuclei of the respective atoms. A Pi (π) bond forms when the orbitals overlap outside the space between the respective nuclei. Now, let’s see the electronic configuration of both the atoms forming the molecule SO3.
Electronic configuration of S :1s22s22p63s23p4
Electronic configuration of O :1s22s22p4
Now, in order to form covalent bonds with three oxygen atoms, sulphur atoms undergo hybridization. The new electronic configuration of the excited sulphur atom will be:
Excited state electronic configuration of S: 1s22s22p63s13p33d2
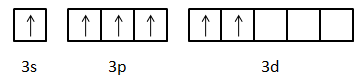
Now, we can see that there are six unpaired electrons are there in the valence orbit of the sulphur atom.
-One electron of s-orbital and two electrons of p-orbitals will form σ-bond with three oxygen atoms. Then the remaining one electron of p-orbital and two electrons of d-orbital will form π-bonds with those three oxygen atoms. So, we can draw the structure of SO3 molecule as:
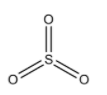
Here, we can see that oxygen will form bonds with its incompletely filled p-orbital. So, we can say that there will be overlapping between the pπ orbitals of sulphur atoms and pπ orbitals of oxygen atoms. So, there will be the presence of pπ−pπ overlapping.
-There are unpaired electrons in the d-orbitals present in the excited state of the sulphur atom. So, there will be overlapping of dπ orbitals of sulphur and pπ orbitals of oxygen. So, we can also say that dπ−pπ overlapping will be present in this molecule.
Therefore the correct answer is (D).
Note: Do not forget that orbitals of sulphur atoms undergo hybridization in the formation of SO3. In the formation of SO3, a total of three σ-bonds and three π-bonds are there.
