Question
Question: Which type of isomerism is shown in\(2,3 - {\text{dichlorobutane}}\) ? A) Structural B) Geometric ...
Which type of isomerism is shown in2,3−dichlorobutane ?
A) Structural B) Geometric C) Optical D) Diastereo
Solution
Hint : -In 2,3−dichlorobutane, the carbon on 2nd and 3rd position are chiral carbons(the carbon atom containing four different groups as its substituents).When we draw the structures of isomers, we also see that the images are mirror images of each other but they are not super imposable.
Complete Step By Step Answer:
2,3−dichlorobutane shows optical isomerism as it fulfills the following conditions-
1. The compound should have chiral carbons.
Ex-It should contain different atoms as its substituent like

2. The isomers must have non-super imposable mirror images.
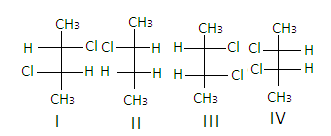
It contains chiral carbons in 2nd and3rd positions as all the four groups attached to the carbons are different and it forms 4 optical isomers which are non -super imposable mirror images of each other.
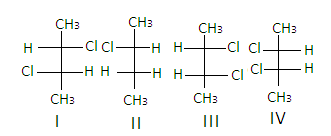
Hence, the correct answer is ‘C’.
Additional Information:
Let us also understand about other types of isomerism-
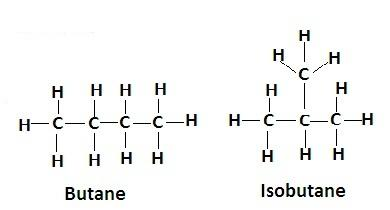
1. Structural isomerism is the isomerism in which the molecular formula is the same but the structure is different as the atoms have different arrangements. Ex- butane and isobutane have same molecular formula but their structures are different-
2. Geometrical isomerism is the isomerism in which spatial arrangement of ligands is changed. When the identical ligands occupy adjacent position it is called cis-isomer and when they are opposite to one other, it is called Trans isomer. It is also called cis-tans isomerism. Ex-
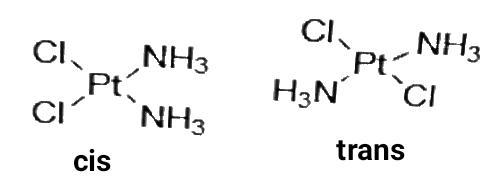
3. Diastereo isomerism is like optical isomerism but the isomers are not mirror images in this isomerism.
In the structure of2,3−dichlorobutane, the structures I and III are diastereomers.
Note :
Optical isomers are the compounds having the same molecular formula but different spatial arrangement of atoms .The isomers form non-super imposable mirror images called enantiomers.
The mirror images when put upon each other are not superimposed because the arrangement of atoms differs.
