Question
Question: Which type of alcohol can exhibit chain isomerism? (A) The alcohol has one carbon atom in the stru...
Which type of alcohol can exhibit chain isomerism?
(A) The alcohol has one carbon atom in the structure.
(B) The alcohol has two carbon atoms in the structure.
(C) The alcohol having three carbon atoms is structured.
(D) The alcohol having four or more than four carbon atoms in structure.
Solution
Chain isomers are compounds which have the same molecular formula but differ in this structure.
They have different numbers of carbon atoms in the main chain.
Alcohols are organic compounds having common formula R−OH.Where R is alkyl group.
Step by step answer: Let us first discuss different types of isomerism present in alcohol.
There are three types of structural isomerism in alcohol.
Therefore, chain isomerism, position isomerism and functional group isomerism.
Let us discuss one by one example:
(I) One carbon: One carbon does not form a chain.
Example: CH3OHmethyl alcohol.
(II) Two carbon: Two carbon form chains but there is only one way to form chains.
Ethyl alcohol.
CH3CH2OH
(III) Three carbon: Three carbon atoms from chain but only one way type shows position isomerism not chain isomerism.
CH3−CH2−CH2−OH
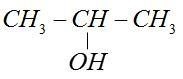
(IV) Four carbon: Alcohol with four carbon atoms can show chain isomerism. Its molecular formula CF4H10O.
We can represent this as:
CH3−CH2−CH2−CH2−OH
N-butyl alcohol
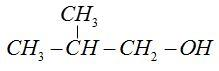
Isobutyl alcohol
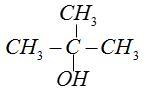
T-butyl alcohol
Therefore, from the above explanation the correct option is (D) The alcohol having four or more than four carbon atoms in structure.
Note: For formation of chain at least four carbon atoms are present in the molecule. Molecules having one two or three carbon atoms just for straight chain and not branches
