Question
Question: Which sublevel is filled just before 5f? (A) 4f (B) 7s (C) 5d (D) 6d...
Which sublevel is filled just before 5f?
(A) 4f
(B) 7s
(C) 5d
(D) 6d
Solution
Read the Aufbau’s principle to obtain the answer for this question. You can also take a look at the periodic table to see which group is filled before the 5f orbital.
Complete step by step answer:
-According to Aufbau’s principle, ‘In the ground state of the atoms, the orbitals are filled with electrons in the order of their increasing energies.’
-Orbitals are filled in the order of increasing value of (n+l). For example, for 3d, n=3 and l=2, so, (n+l)=5 and for 4s, n=4 and l=0, so, (n+l)=4. As the value of (n+l) for 4s orbital is less than that of 3d orbital, 4s orbital is filled before 3d orbitals.
-Similarly, let us calculate (n+l) values for all the orbitals given in the question.
For 5f orbital, n=5 and l=3, so (n+l)=8
For 4f orbital, n=4 and l=3, so (n+l)=7
For 7s orbital, n=7 and l=0, so (n+l)=7
For 5d orbital, n=5 and l=2, so (n+l)=7
For 6d orbital, n=6 and l=2, so (n+l)=8
-If two orbitals have the same value of (n+l), then the orbital with lower value of n will be filled first. Now here, 4f has the least n-value so it will be filled first. Then comes, 5d and then 7s orbitals followed by 5f orbital and then 6d orbital.
-Aufbau’s diagram is shown below:
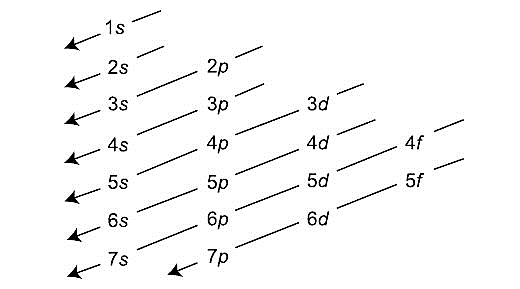
-Therefore, we can conclude that 7s-orbital is filled just before 5f-orbital.
So, the correct answer is “Option B”.
Note: Learn the Aufbau schematic diagram. Remember according to the rules, we need to check (n+l) values first and then if they are the same, then we go for an orbital having lower n-value.
