Question
Question: Which statement is true about the most stable Lewis structure for \(C{S_2}\)? A) There are no lone...
Which statement is true about the most stable Lewis structure for CS2?
A) There are no lone pairs in molecules.
B) All bonds are double bonds.
C) The central atom does not have an octet of electrons.
D) A sulfur atom must be the central atom for the structure to be stable.
Solution
We all know Electron dot structure:
A Lewis dot diagram could also be a representation of the valence electrons of an atom that uses dots around the symbol of the element. The quantity of dots equals the quantity of valence electrons within the atom.
Example:
The electron of Mg is 2 it are often represented in electron dot structure as,
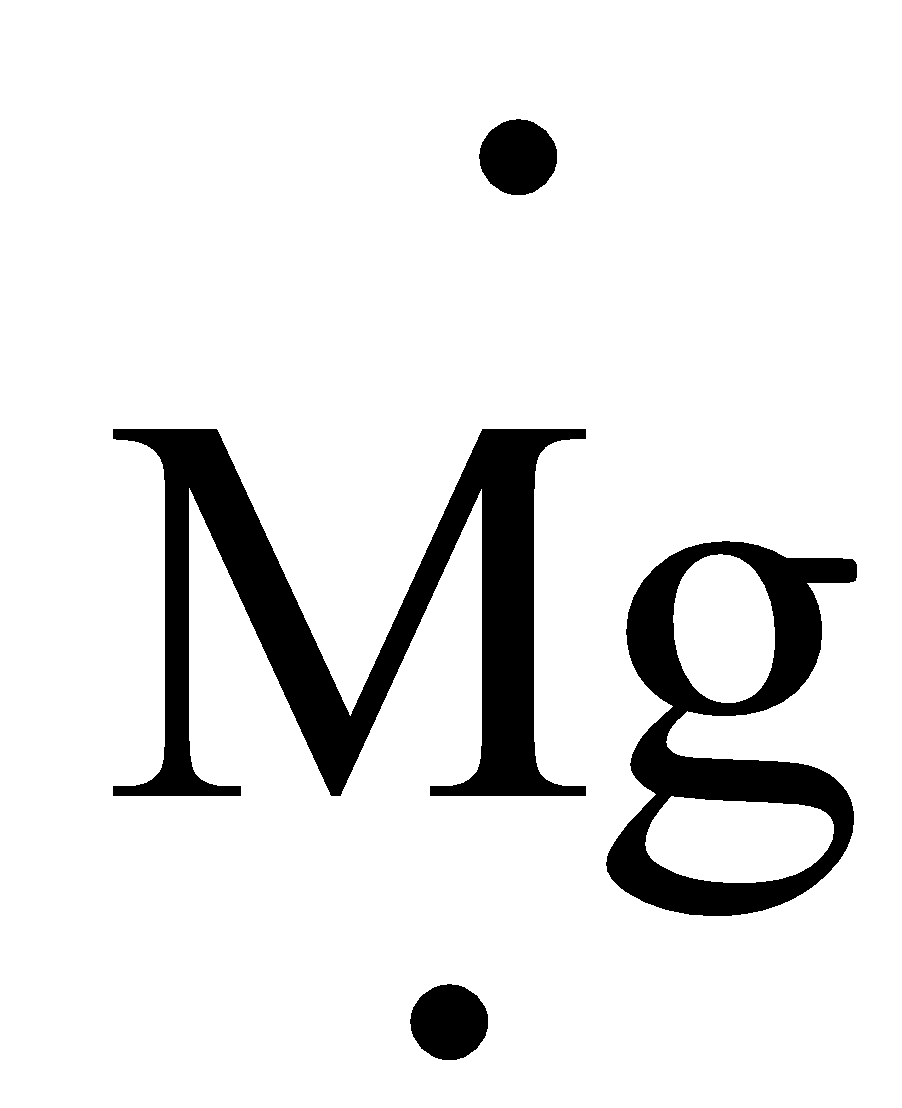 .
.
Complete step by step answer:
We know that the Lewis structure is the simple representation of the amount of valence electrons during a molecule. To draw the Lewis structures of a molecule first calculate the entire number of valence electrons within the atom.
There are valence electrons for the Lewis structure. Carbon is the smallest amount of electronegative atom and goes within the middle of this structure. The Lewis structure requires you've double bonds between the carbons and sulfur atoms to fill the octet of carbon.
The Lewis structure of is,
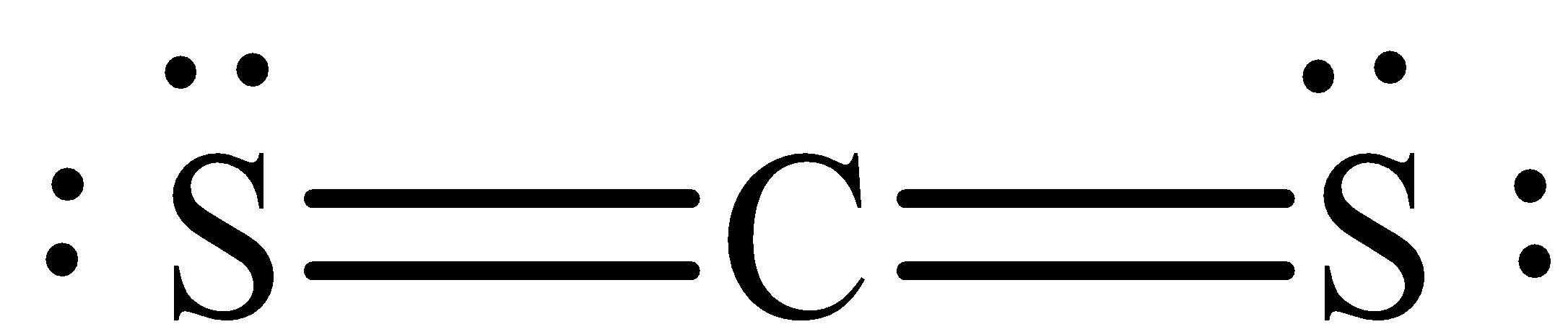
Therefore, the correct option is B. .
Note: We can define transfer of electrons as the process during which an electron shares one or more electrons to its neighboring atom. We all know that there must be eight electrons within the outermost orbital of an atom. This is often referred to as octet rule. If an atom has but eight electrons, they have a tendency to react and yield stable compounds.
We can state octet rule, as “An atom is more stable when their outermost shells are crammed with eight electrons”. Molecules like halogens, oxygen, nitrogen and carbon obey the octet rule. All the weather of the most groups obeys the octet rule.
We know that there are two main sorts of bonds. They are,
- Ionic bonds
- Covalent bonds
Ionic bonds are formed thanks to the transfer of electrons from one atom to another. This generally happens in metal. Ionic compounds such as common salt, potassium chloride; saltpeter, salt etc have ionic bonding in between their atoms.
