Question
Question: Which species has the same shape as the \(N{{O}_{3}}^{-}\)? A. \(S{{O}_{3}}\) B. \(S{{O}_{3}}^{2...
Which species has the same shape as the NO3−?
A. SO3
B. SO32−
C. ClF3−
D. ClO3−
Solution
The atoms which are isoelectronic in nature will share the same properties i.e. they will have the same number of electrons, same shape and other properties.
Complete step by step solution:
As per the given question we have to find out which species will share the same shape with NO3−. So, as we know in chemistry the species having the same number of electrons (i.e. if they are isoelectronic in nature) in their structure will share the same properties which mean both the species will have the same shape, atomic size, bonds, and other properties. So, Here, the number of electrons NO3− will have is (7+3×8)+1=32electrons in total. Likewise, we have to find out the number of electrons of the given species so as to see which species is isoelectronic with NO3−.
Thus, The number of electrons SO3 will have is 32.
The number of electrons SO32− will have is 34.
The number of electrons ClF3− will have is 45.
The number of electrons ClO3− will have 42.
So, we can see that SO3is having the same electrons as the NO3−. Thus, SO3 will have the same shape as the NO3−. In nitrate ion i.e. NO3−, where it share a sp2 type of hybridisation and there is one central atom i.e. nitrogen which is surrounded by three identically bonded oxygen atoms that lie at the corners of a triangle and at the same one-dimensional plane. It has no lone pairs and has three electron domains. Hence, nitrate ion molecular geometry is slightly bent giving a trigonal planar structure.
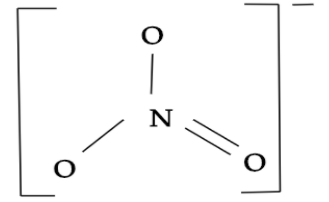
While, sulphur trioxide i.e. SO3 also shares a sp2 type of hybridisation and here sulphur will be the central atom which is surrounded by three identically bonded oxygen atoms. It has no lone pairs and the molecular geometry it has is trigonal planar.
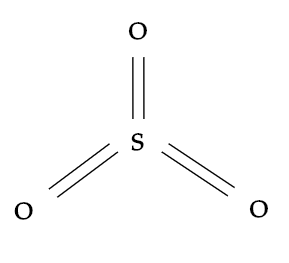
Hence, the correct option is A.
Note: Species sharing the same hybridisation and number of electrons will have the same molecular geometry. As well as, those species who are isoelectronic in nature will have the same properties like atomic radii, melting point, boiling point, etc.
