Question
Question: Which sequence of steps describes the best synthesis of \(2-phenylpropane\) ? (A) Benzene \(+\) \(...
Which sequence of steps describes the best synthesis of 2−phenylpropane ?
(A) Benzene + 2−chloropropane, AlCl3
(B) 1.Benzaldehyde (C6H5CH=O)+CH3CH2MgBr, diethyl ether
2.H3O+
3.H2SO4, heat
(C) 1.Bromobenzene + Mg , diethyl ether
2.Propanol (CH3CH2CH=O)
3.H3O+
4.H2SO4, heat
(D) 1.Bromobenzene +Mg, diethyl ether
2.Acetone [(CH3)2C=O]
3.H3O+
4.H2SO4, heat
Solution
We know that the both types of react Acetyl Chloride Benzene ions, Friedel craft alkylation and Friedel craft acylation involve electrophilic aromatic substitution. Friedel crafts alkylation reaction – The reaction involves Alkylation of aromatic rings by alkyl halide in presence of Lewis acid is called Friedel craft alkylation reaction. In these reactions Lewis acids such as aluminum chloride and iron chloride are used. Friedel craft alkylation reaction can be represented in short form as follows;
Aromatic ring + Alkyl halide Lewis acid→ Alkyl aromatic compound
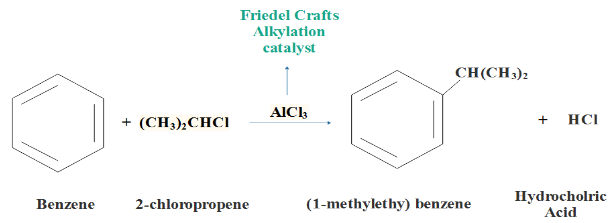
in this reaction aromatic ring here is benzene; alkyl halide Lewis acid is 2−chloropropane and reagent here will be AlCl3
Complete step-by-step answer: Friedel Craft alkylation reaction involves use of alkyl group (R). We know that reactions of different molecules with carbon of benzene, but it is also possible to form carbon-carbon bonds. It requires alkyl halides to react with benzene in presence of catalyst such as a Lewis acid thus an example of Electrophilic substitution reaction can be a chloromethane which reacts with benzene in presence of aluminum chloride as well as iron chloride. The Lewis acids make it easy for chlorine atoms to leave bond by weakening bond. Although the product of a reaction has high nucleophile strength.
Friedel crafts alkylation of Benzene : On treating benzene with alkyl halide, in presence of Lewis acid such as anhydrous aluminum chloride, it forms alkyl benzene. This reaction is known as Friedel craft alkylation reaction.
Friedel crafts alkylation reaction mechanism it takes place in 3 steps:
Step 1. Formation of an electrophile (methyl carbonium).
Step 2. Electrophile methyl carbonium attacks on benzene (aromatic compound) and forms a cationic complex as an intermediate which is stabilized by resonance.
Step 3. Removal of H+ ion or proton takes place from the complex formed above in step 2 and formation of alkyl aromatic compound (toluene) takes place through electrophilic substitution.
These are the steps for Friedel crafts alkylation reaction
This mechanism mainly involves three fundamentals. There is a formation of a new pi bond from carbon double bond, removal of a proton from the carbon-hydrogen bond, and reformation of carbon double bond. You must understand these two main steps involving electrophilic aromatic substitution reaction mechanism. The first step initiates the attack of an electrophile on the benzene ring. After that, initial attack helps the formation of uranium ions by gaining positive charge or protons. Subsequently, the entire process is slow due to electrophile taking its time attacking the aromatic ring.
Since the aromatic ring loses its aromatic it results in the release of high activation energy. Several factors, such as steric hindrance, probability, and resonance, play a crucial role in the electrophilic attack. And the second step involves the removal of a proton from the ion by a weak base. This removal occurs due to the attack of a weak base on the formed carbocation. Then the aromatic is stored again by the formation of the pi bond via electrons. The entire process is relatively fast. One key thing to remember is that due to the attack of the electrophile, carbocation loses a proton in the process.
Therefore, correct answer is option (A) Benzene + 2−chloropropane, AlCl3
Note: Note that the reaction doesn’t take place if benzene has a substituent group that is more deactivating than halogens. Aryl and vinyl halides can’t be used in this reaction as their carbocations are very reactive and highly unstable. Acylation reactions generally form only ketones
