Question
Question: Which sample A or B has a shorter mean life?...
Which sample A or B has a shorter mean life?
Solution
Mean life, in radioactivity, average lifetime of all the nuclei of a particular unstable atomic species. This time interval may be thought of as the sum of the lifetimes of all the individual unstable nuclei in a sample, divided by the total number of unstable nuclei present.
Complete step-by-step solution:
The mean life of B is shorter than that of A
According to the Radioactive Decay Law states that the decay rate of a nucleus is given by the formula of
dtdN=−λN
Where,
dtdN is the rate of decay of the nucleus with respect to time, λ is the decay rate constant of the nucleus and N is the number of nuclei present in the sample. As shown in the graph the X axis the decay rate and Y axis is the time of decay, now to point the decay rate constant in the graph we have to draw a slope on the curve of sample A and B like this
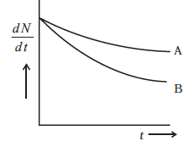
The slope of the A and B curve dedicates the decay rate constant of the sample A and B respectively. Now as for the mean life of the samples, the mean life formula is
τ=λ1
Where,τis the mean life and λ is the decay rate constant. So according to the diagram the mean life of A and B are given as
τA=λA1,τB=λB1
λA<λB
τA>τB
Now as we can see in the above diagram (in the explanation), the value of decay rate constant of B and A is λA<λB (the flatter the curve the lesser is the slope therefore, the value of the slope of B is greater than A as the curve of B is greater than A) respectively. Hence, due to inverse proportionality the value of mean life of B is lesser than that of A.
Therefore, the mean life of B is shorter than that of A.
Note: We need to remember that radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive.
