Question
Question: Which reagent is used for the following conversion? 
A. Na
B. aq. KOH
C. alcoholic KOH followed by NaNH2
D. Zn
Solution
The dihalide is geminal in nature, as both the halo groups are attached to one carbon atom only. The question will be easy to solve if we go through the mechanism method. Sodium metal extracts acidic hydrogen, aq. KOH adds −OH group replacing halo group, alcoholic KOH followed by NaNH2 are reagents of elimination and zinc removes halo groups only.
Complete answer:
Let us discuss this conversion through its mechanism using a general reaction at first;
If the halogens are attached to the same carbon (geminal) or adjacent carbon atom (vicinal) and it reacts with base, the base extracts the hydrogen atoms and halogen atoms along with it. The general reaction of dihaloalkane elimination is
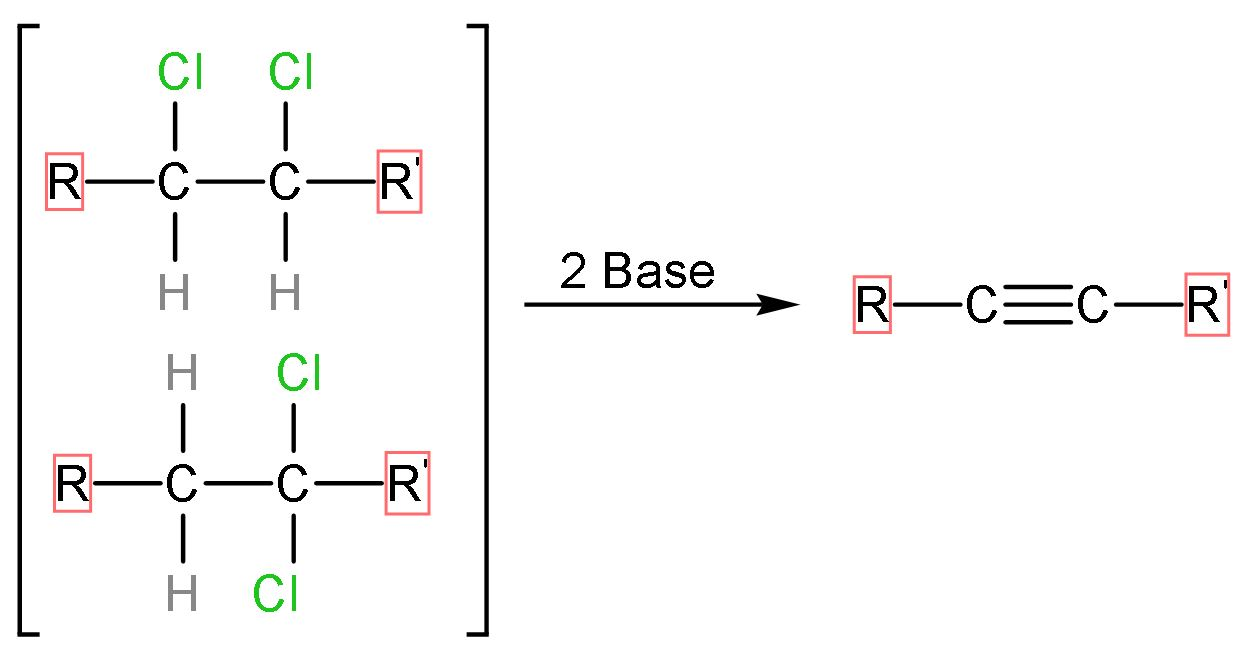
Let us see the mechanism of this question,
Step (1)- The dihaloalkane is reacted with alcoholic KOH, the halogen attached to it and the hydrogen atom from the adjacent carbon atom will be removed by it as alc. KOH is a strong base. The hydrochloric acid is removed and a double appears replacing the atoms. The reaction follows as
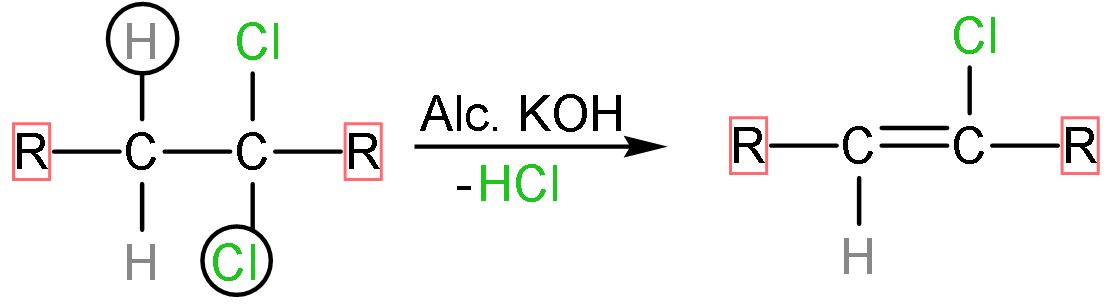
Step (2)- The forward is reaction carried out by NaNH2 which is very strong base, as it denotes its lone pair and negative charge to incoming electrophiles. The hydrogen atom and chlorine atom present on adjacent carbon atoms are removed by adding one more bond between the two which is π- bond. The reaction looks like,
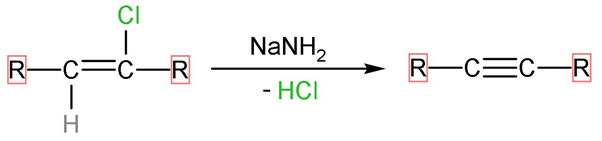
The correct answer to this question is ‘b’. Alcoholic KOH followed by NaNH2 reagent is used for the following conversion.
Note:
The moles of bases required to convert dihaloalkanes to terminal (in which triple bond is between C1−C2) alkynes are 3 not 2. The terminal alkynes are acidic in nature, as they readily react with bases to remove acidic hydrogens from them. RC≡CH+Base→RC≡C−base++21H2. As, one of the base molecules will remove the terminal hydrogen instead of one of the halides.
