Question
Question: Which reagent converts propene to 1-propanol? (A) \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\] (B) \[{{B}_...
Which reagent converts propene to 1-propanol?
(A) H2O,H2SO4
(B) B2H6,H2O2,OH
(C) Hg(OAc)2,NaBH4/H2O
(D) Aq.KOH
Solution
The structure of propene is as follows.
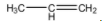
The structure of 1-Propanol is as follows.
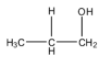
In the structure of 1-propanol, the alcohol group is present on the terminal carbon atom.
Normal reagents react with propene and form 2-propanol as the product.
We need selective chemicals to get 1-propanol from propene.
Complete step by step solution:
-In the question, it is asked which chemical is going to convert propene into 1-propanol.
-Coming to given option, option A H2O,H2SO4.
-The reaction of propene with H2O,H2SO4is as follows.
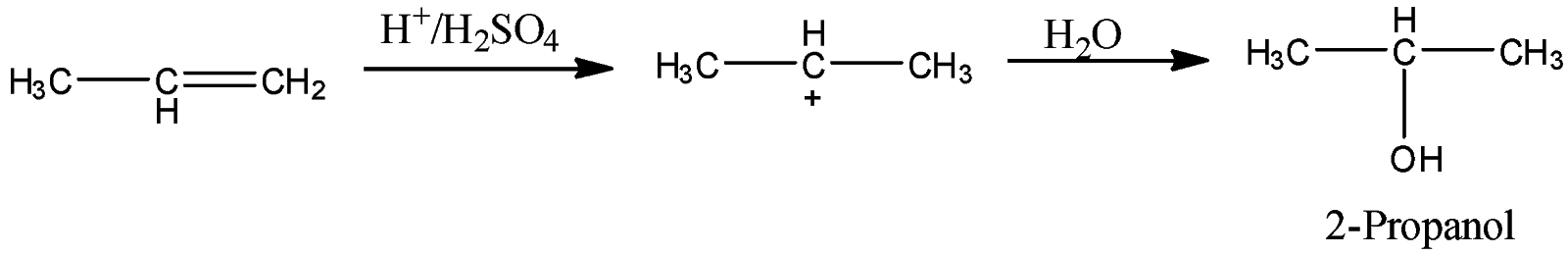
-Propene reacts with H2O,H2SO4and forms a product called 2-propanol. So, Option A is wrong.
-Coming to option B, B2H6,H2O2,OH.
- The reaction of propene with B2H6,H2O2,OHis as follows.
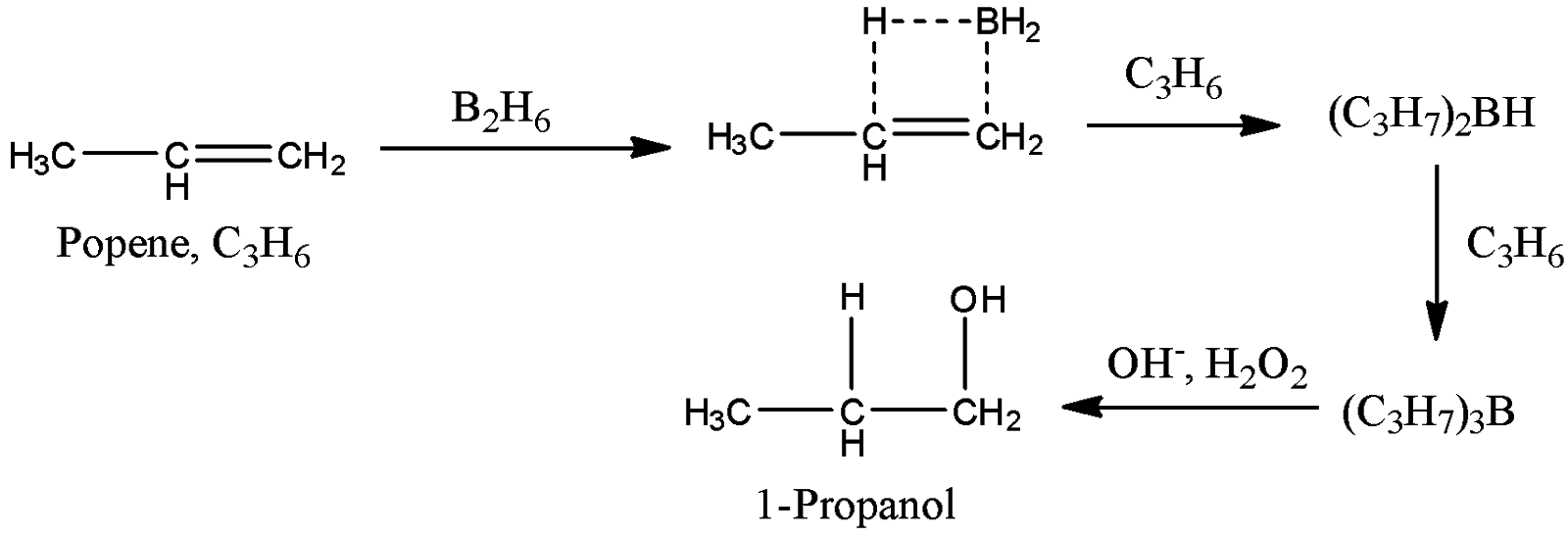
- Propene reacts with B2H6,H2O2,OHand forms 1-propanol as a product. Therefore option B is correct.
-Coming to option C, Hg(OAc)2,NaBH4/H2O
-The reaction of propene with Hg(OAc)2,NaBH4/H2O is as follows.
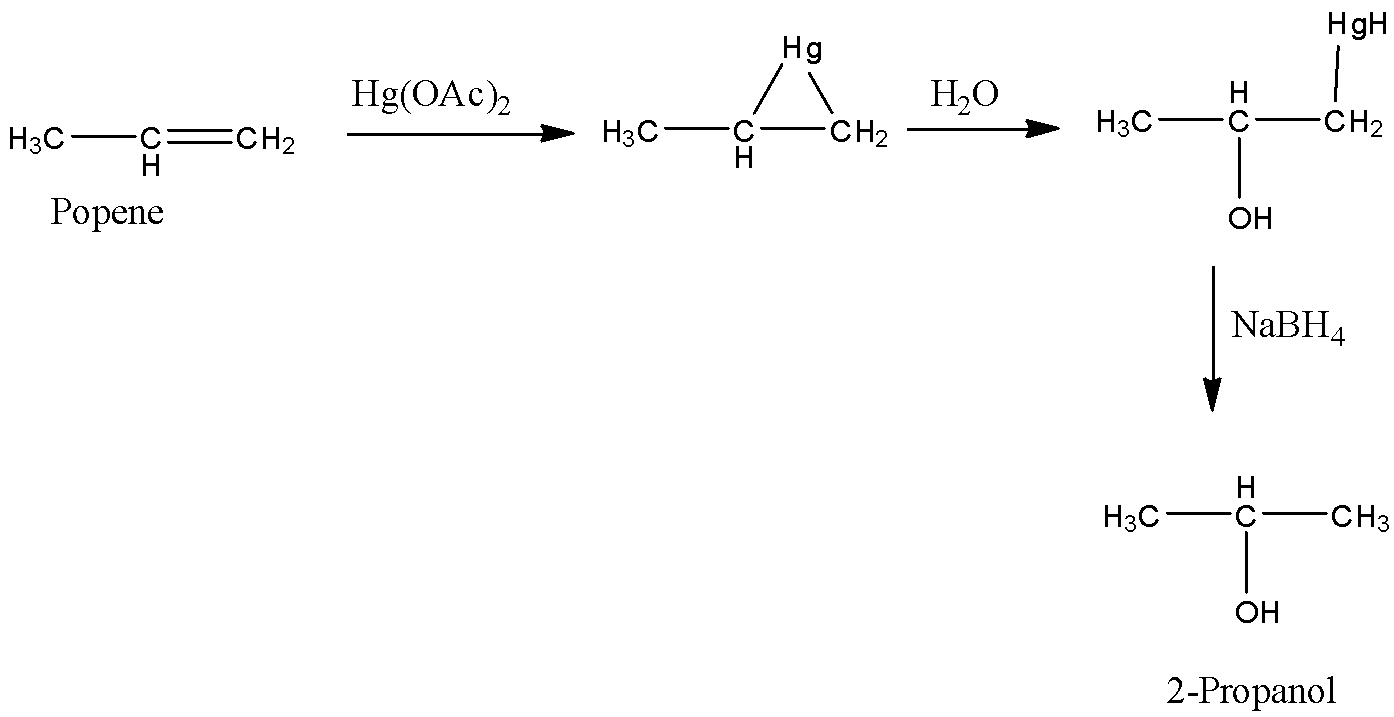
- Propene reacts withHg(OAc)2,NaBH4/H2O and forms a product called 2-propanol. So, Option C is wrong.
- Coming to option D, Aq. KOH.
- The reaction of propene with Aq. KOH is as follows.
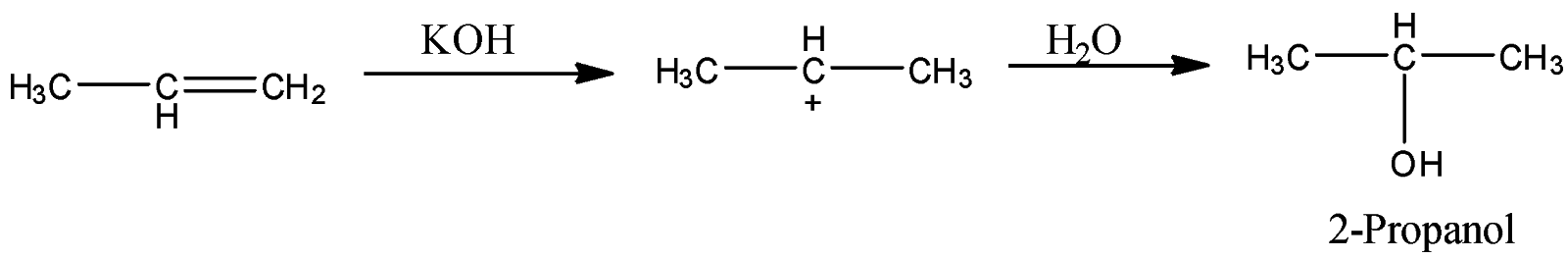
- Propene reacts with Aq. KOH and forms a product called 2-propanol. So, Option D is also wrong.
-Therefore reagentB2H6,H2O2,OH converts propene to 1-propanol.
So, the correct option is B.
Note: The reaction of propene with B2H6,H2O2,OHgives 1-propanol as the product. This reaction is called hydroboration. Hydroboration gives terminal alcohols as the product when reacts with an alkene. Hydroboration is a selective reaction to prepare terminal alcohols from alkenes.
