Question
Question: Which reagent can be used to distinguish between 1-butyne and 2-butyne? A.Alc. KOH B.\[Alc.{\tex...
Which reagent can be used to distinguish between 1-butyne and 2-butyne?
A.Alc. KOH
B.Alc. KMnO4
C.Br2, water
D.Ag+
Solution
Since, 1-butyne is a terminal alkyne, it reacts with tollen’s reagent whereas 2-butyne does not react as it is an internal alkyne. So, ammoniacal silver nitrate is used to distinguish between end alkyne and other alkyne as it reacts with acidic hydrogen.
Complete answer:
The structures of two given alkynes in the question, 1-butyne and 2-butyne are shown below:
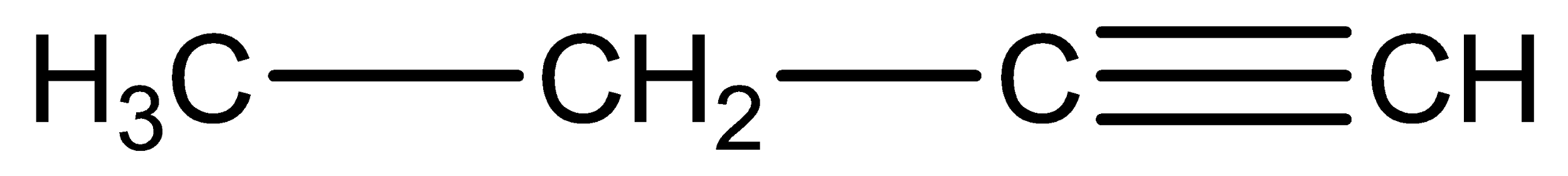
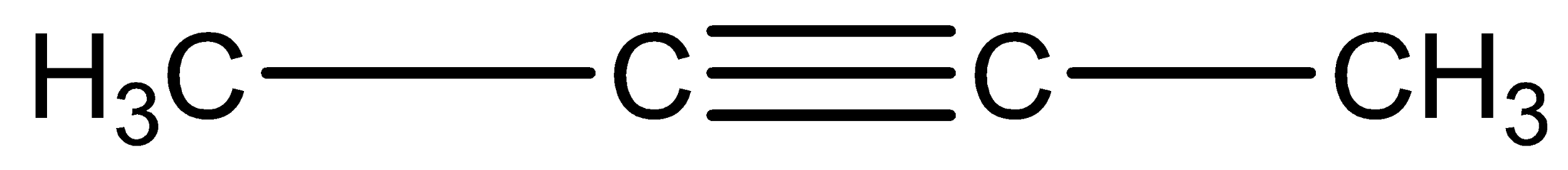
Alkynes can undergo a number of additional reactions due to the presence of two pi-bonds in which alkynes can add two molecules of the reagent which is so unlike alkene as they can only add one molecule of reagent due to the presence of only one pi-bond.
Tollen’s reagent is a chemical reagent that consists of a solution of silver nitrate, ammonia and sodium hydroxide. Because of the acidic nature of the hydrogen (H) atoms attached to the triple bonded carbon (C), terminal alkynes react with heavy metallic salts such as the silver nitrate. Ammoniacal silver nitrate is used to distinguish between end alkyne and other alkyne as it reacts with acidic hydrogen.
Only the end alkyne has acidic hydrogen so it reacts with 1-butyne only. So, it distinguishes between them.
Therefore, the correct answer is option (D).
Note: In the reaction with Alc. KMnO4solution under the neutral conditions, alkynes react with dilute KMnO4 and give diketones. Whereas, on reaction with basic KMnO4, any alkyne undergoes oxidative cleavage at high temperature. So, we cannot distinguish 1-butyne and 2-butyne.
When bromine solution is added to the alkyne, the reddish brown colour disappears as the product is colorless. Thus, both 1-butyne and 2-butyne decolorize bromine water solution. Hence, Br2 water does not distinguish between 1-butyne and 2-butyne.
