Question
Question: Which reaction will occur at the fastest rate? 
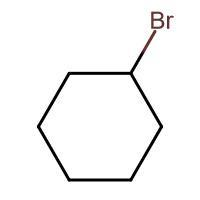
(A) HBr
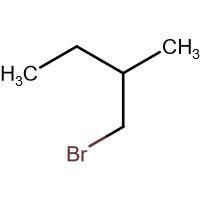
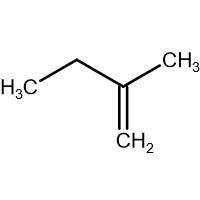
(B) HBr
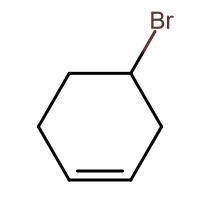
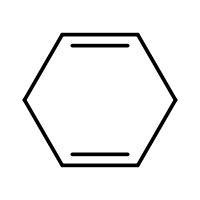
(C) HBr
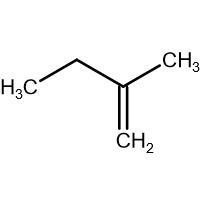
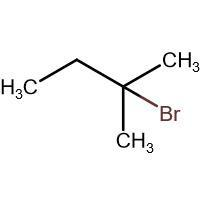
(D) HBr
Solution
The addition of HBr in absence of any catalyst follows markovnikov's rule of addition, so the reagent with the stable intermediate will be the major product and also will occur fast. In this question delocalize the double bonds such that stability is given the preference.
Complete step by step solution:
We will now study the markovnikov's rule of addition before applying it in the reaction. Markovnikov Rule: In an addition reaction of an acid HX (hydrogen chloride, hydrogen bromide, or hydrogen iodide) to an alkene, the halogen atom of HX becomes bonded to the carbon atom that has the most branching i.e. the least number of hydrogen atoms.
Along with this rule, we also have to consider the stability of the intermediate that is formed because the reaction with the most stable intermediate will occur the fastest.
Inductive effect is an electronic effect which results in permanent polarization of the bond in which the two atoms have different values of electronegativity.
We will find the stability of carbocation due to inductive effect:
Primary carbocation:
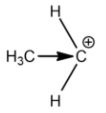
Secondary carbocation:
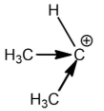
Tertiary carbocation:
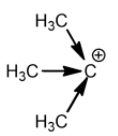
Methyl groups increase the electron density on the carbocation. So, the stability of carbocation is directly proportional to the number of methyl or alkyl groups attached. This is the reason why tertiary carbocation is the most stable carbocation.
We will now see the products of the reaction and also see the degree of carbon to which the halogen atom( Br) is attached to arrive at the answer.
-In (A). the halogen atom(Br) gets attached to a 2∘ carbon and there is no resonance after delocalization of the double bond.
-In (B). the halogen atom(Br) gets attached to a 1∘ carbon and there is no resonance after delocalization of the double bond.
-In (C). the halogen atom(Br) gets attached to a 2∘ carbon and there is no resonance after delocalization of the double bond.
-In (D). the halogen atom(Br) gets attached to a 3∘ carbon and there is no resonance after delocalization of the double bond.
Since all four options do not show resonance in the intermediate, we will follow the stability rule discussed above i.e. the inductive effect.
Through inductive effect, the intermediate formed in compound (D) is most stable because tertiary carbocation is the most stable carbocation.
Therefore, the correct answer is option (D).
Note: While delocalizing the double bond in option C note that only one double bond gets delocalized and not both of them and that is the reason why resonance doesn’t exist. The carbon with the least number of hydrogen atoms is also considered as the most branched, as they mean the same thing.
