Question
Question: Which reaction sequence would be best to prepare 3-chloroaniline from benzene? A.Chlorination, Nit...
Which reaction sequence would be best to prepare 3-chloroaniline from benzene?
A.Chlorination, Nitration, Reduction
B.Nitration, Chlorination, Reduction
C.Nitration, Reduction, Chlorination
D.Nitration, Reduction, Acetylation, chlorination, hydrolysis
Solution
The nitro group is added in the benzene ring to form the nitrobenzene. Then, nitro benzene is treated with ferric chloride. Since the nitro group is a meta director, we can add Chlorine (Cl) through chlorination. After that, we can reduce the NO2 Group to NH2 Group.
Complete answer:
First we add nitro group (NO2) to the benzene ring to form nitrobenzene through nitration of benzene as nitration of benzene will give nitro benzene as a product. Then, we will treat nitro benzene with ferric chloride in the presence of chlorine gas which will give 3- chloro nitro benzene. In the last step, the reduction of nitro group in 3-chloro nitro benzene by lithium aluminium hydride which will give us 3-chloro aniline.
Since the nitro group is a meta director, we can then add Cl through chlorination. After that, we can reduce the NO2 Group to NH2 Group.
Here’s a possible synthesis we can consider:
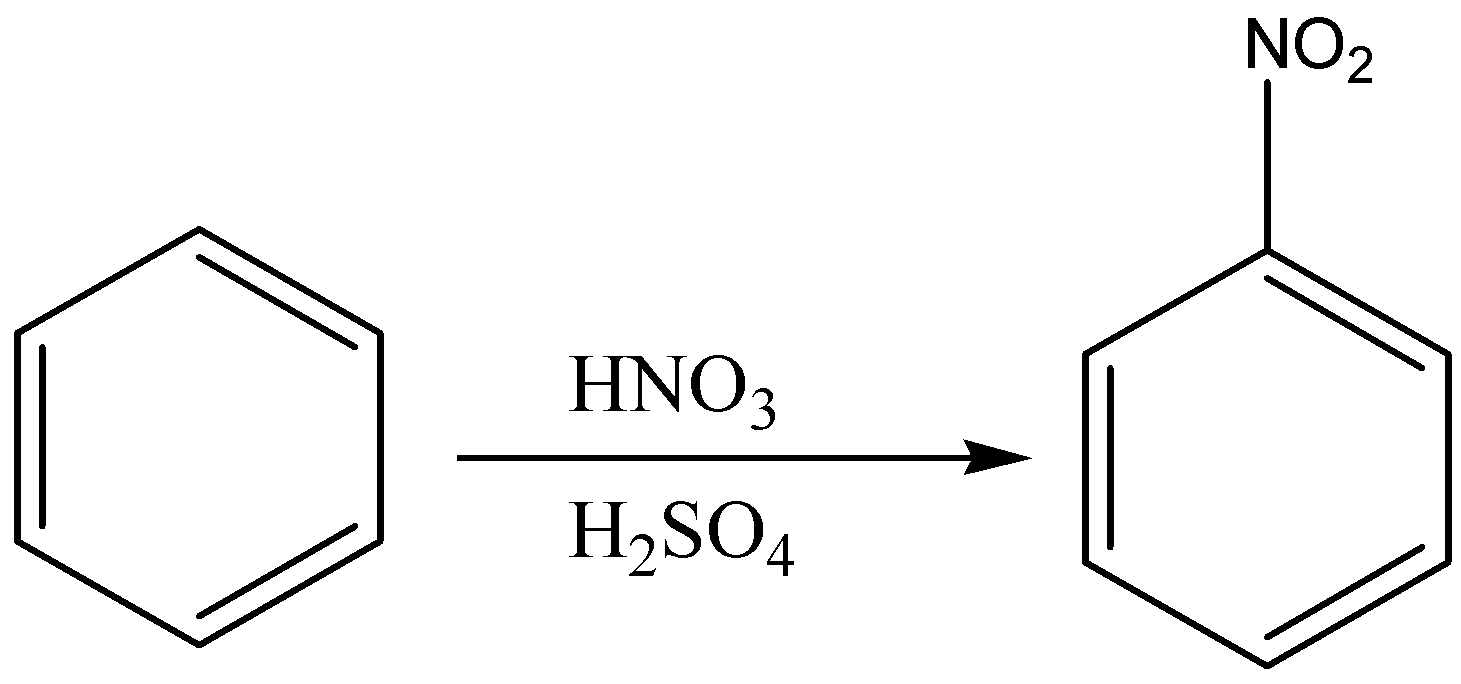
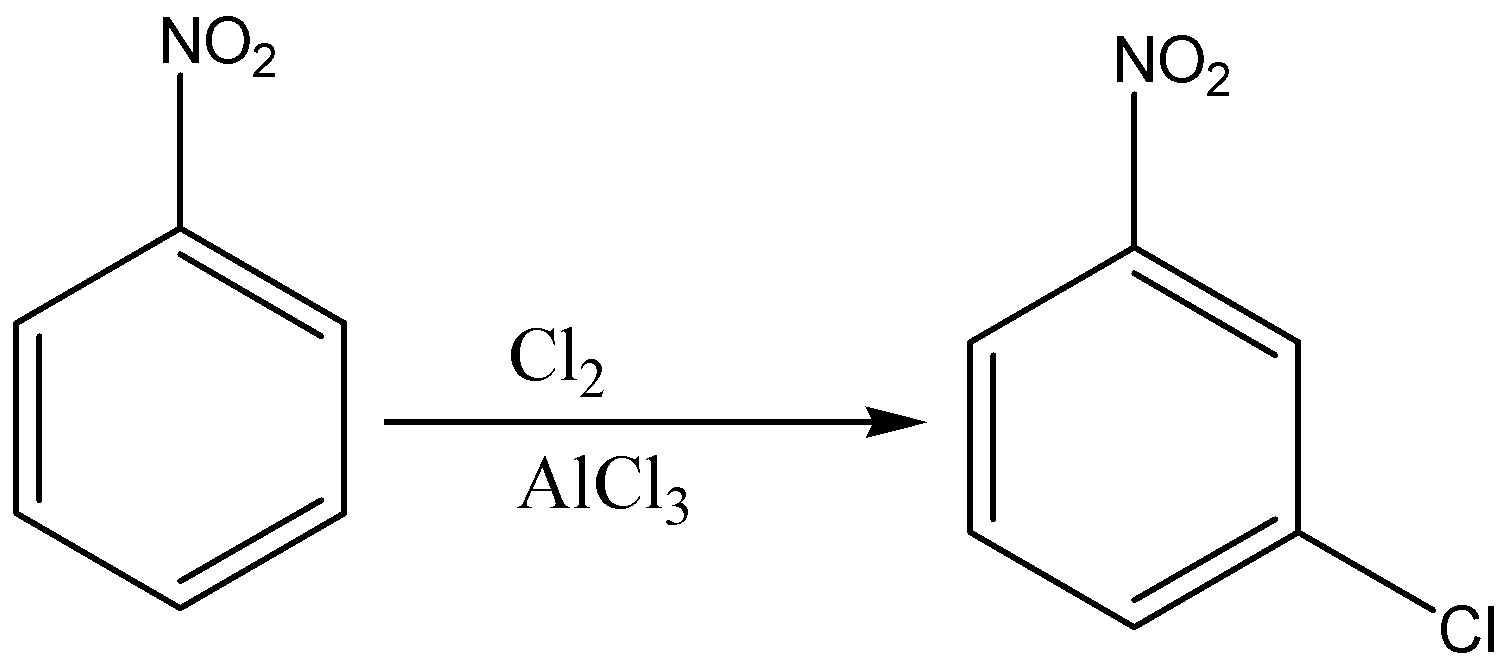
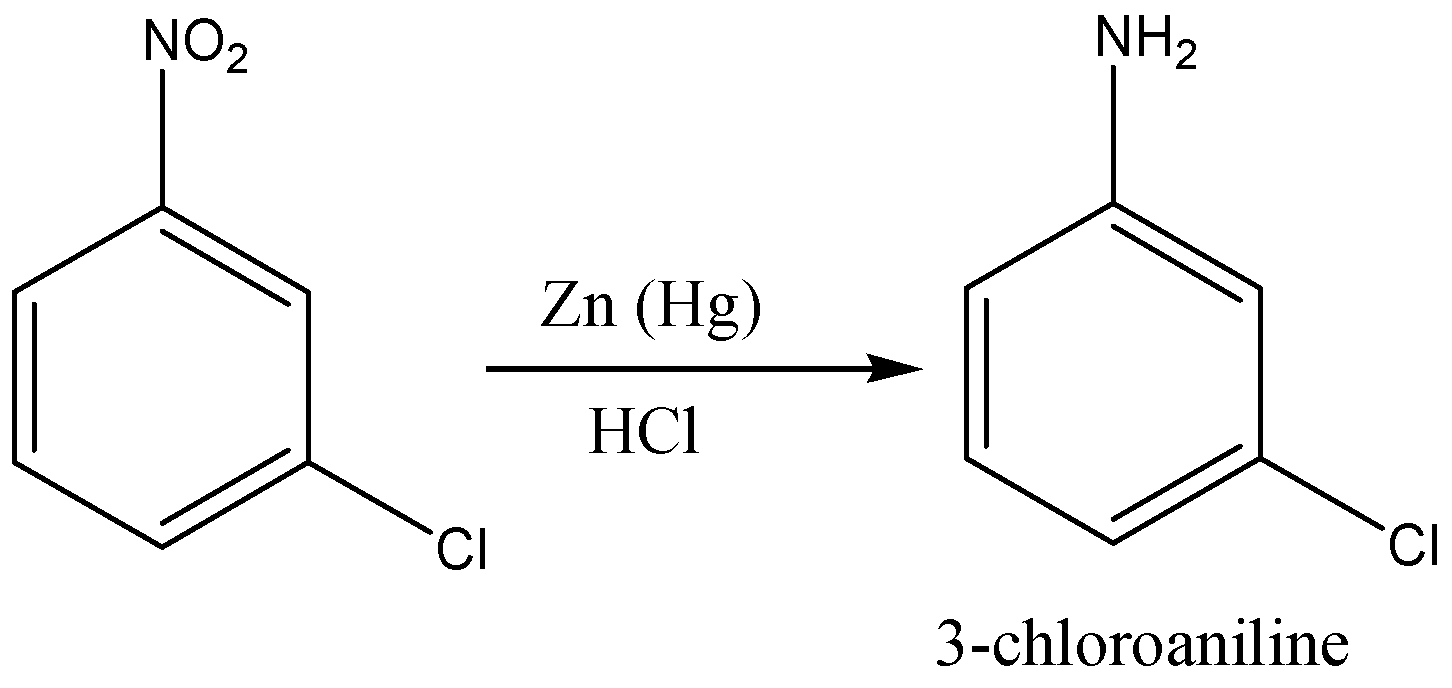
So, the correct reaction sequence that is best to prepare 3-chloroaniline from benzene is Nitration, Chlorination, Reduction.
Therefore, the correct answer is option (B).
Note: An alternative we can consider to prepare 3-chloroaniline from benzene is:
This synthesis involves adding two substituents to the benzene ring. In this case, we need to consider the order of substitution. Cl is an ortho-para director. That means, if Cl is first added to the benzene, the next substituent will be placed in the ortho/para position and that will not work since, in the desired product, NH2 is meta to Cl. That means, we need to add Cl in the last. NH2 is also an ortho-para director, so that means we can’t add Cl after forming aniline.
3-Nitrochlorobenzene is an organic compound with the formula C6H4ClNO2. It is a yellow crystalline solid that is important as a precursor to other compounds due to the two reactive sites present on the molecule.
