Question
Question: Which property of water is used while washing clothes? A) Odor B) Color C) Solubility D) ...
Which property of water is used while washing clothes?
A) Odor
B) Color
C) Solubility
D) Sand
Solution
Hint : Sand is not a property of water. Water is a colorless and odourless liquid. It has many properties like it is dielectric and it is a polar molecule due to which it acts as a good solvent for polar compounds.
Complete Step By Step Answer:
Water is colorless and odorless liquid. It is also a good solvent as it can dissolve other molecules and compounds. Water makes an excellent solvent for two reasons-
due to its polarity
And its ability to form hydrogen bonds with dissolved ions of molecules.
Water is a highly polar molecule. It is because the high electro-negativity difference between oxygen atom and hydrogen atom creates partial positive charge on oxygen atom and partial negative charge on hydrogen atom resulting in unequal distribution of electron density throughout the molecule. Due to separation of these charges, an electric dipole develops between the ions of water. This makes the water dielectric. When the clothes are washed –
The detergent or soap becomes easily soluble in water forming lather.
This lather mixes with the dirt and dust particles.
The separated ions of dust particles become surrounded with oppositely charged ends of water dipoles and hydrated. So they get washed away.
Hence, the solubility property of water is used while washing clothes.
The Correct answer is C.
Additional Information:
The molecular formula of water is H2O and its structure is bent overall because oxygen carries two lone pair electrons. The structure is given as-
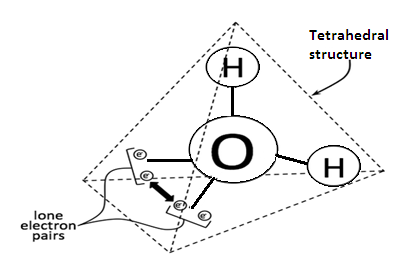
It is a polar molecule hence it is good at dissolving ions and polar molecules but not good at dissolving non-polar molecules.
Note :
The reason for the polarity of water molecule is-
Water has one oxygen atom in center and two hydrogen atoms on either side of oxygen atoms.
The bond between oxygen and hydrogen atom is strong and one molecule of water forms hydrogen bond with other molecules of water.
Due to differences in electronegativity, partial positive charge forms on hydrogen atoms and partial negative charge forms in oxygen atoms [as oxygen is more electronegative than hydrogen].
