Question
Question: Which product is not formed by intermolecular aldol condensation? ...
Which product is not formed by intermolecular aldol condensation?
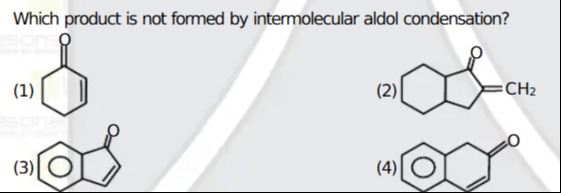
A six-membered ring (cyclohexene) with a carbonyl group (=O) at the 1-position and a double bond between the 3- and 4-positions.
A bicyclic structure. One ring is a six-membered ring (cyclohexane) fused to a five-membered ring (cyclopentanone). The cyclopentanone has an exocyclic double bond to a CH2 group.
A bicyclic structure. A six-membered aromatic ring (benzene) is fused to a five-membered ring (cyclopentanone). The carbonyl group (=O) is on the five-membered ring.
A bicyclic structure. A six-membered aromatic ring (benzene) is fused to a six-membered ring (cyclohexenone). The cyclohexenone has a carbonyl group (=O) at the 1-position and a double bond between the 3- and 4-positions.
Option (3)
Solution
Out of the four products shown, three can be obtained from intermolecular aldol condensation (often followed by subsequent steps such as dehydration or Michael addition as seen in the Robinson annulation). The product in option (3) is a benzofused cyclopentanone (an indanone‐type structure) whose formation instead requires an intramolecular cyclization (typically via Friedel–Crafts acylation or intramolecular aldol condensation) rather than an intermolecular aldol reaction.
